CH. 11 Sec. 1
... a. Bohr did not agree with his theory. b. Dalton proved Democritus wrong. c. Aristotle did not agree with his theory. d. Rutherford proved Democritus wrong. From Aristotle to Modern Science ...
... a. Bohr did not agree with his theory. b. Dalton proved Democritus wrong. c. Aristotle did not agree with his theory. d. Rutherford proved Democritus wrong. From Aristotle to Modern Science ...
Directed Reading B Section: Development of the Atomic Theory
... a. Bohr did not agree with his theory. b. Dalton proved Democritus wrong. c. Aristotle did not agree with his theory. d. Rutherford proved Democritus wrong. From Aristotle to Modern Science ...
... a. Bohr did not agree with his theory. b. Dalton proved Democritus wrong. c. Aristotle did not agree with his theory. d. Rutherford proved Democritus wrong. From Aristotle to Modern Science ...
Standard Enthalpy of Formation
... tabulated thermodynamic data for reactants and products. However, the laws of thermodynamics only allow us to measure changes in enthalpy, internal energy and entropies (ΔH, ΔU and ΔS, not the absolute values of U, H, and S, and we cannot tabulate absolute enthalpies of substances. Instead, we can ...
... tabulated thermodynamic data for reactants and products. However, the laws of thermodynamics only allow us to measure changes in enthalpy, internal energy and entropies (ΔH, ΔU and ΔS, not the absolute values of U, H, and S, and we cannot tabulate absolute enthalpies of substances. Instead, we can ...
Chapter 2 "Elements, Atoms, and the Periodic Table"
... The hardest material in the human body is tooth enamel. It has to be hard so that our teeth can serve us for a lifetime of biting and chewing; however, tough as it is, tooth enamel is susceptible to chemical attack. Acids found in some foods or made by bacteria that feed on food residues on our teet ...
... The hardest material in the human body is tooth enamel. It has to be hard so that our teeth can serve us for a lifetime of biting and chewing; however, tough as it is, tooth enamel is susceptible to chemical attack. Acids found in some foods or made by bacteria that feed on food residues on our teet ...
NSCC Chem 121 chapter2
... electrical charge and have a mass of 1 atomic mass unit (u). • Neutrons are located in the nucleus of an atom. They carry no electrical charge and have a mass of 1 atomic mass unit (u). • Electrons are located outside the nucleus of an atom. They carry a -1 electrical charge and have a mass of 1/183 ...
... electrical charge and have a mass of 1 atomic mass unit (u). • Neutrons are located in the nucleus of an atom. They carry no electrical charge and have a mass of 1 atomic mass unit (u). • Electrons are located outside the nucleus of an atom. They carry a -1 electrical charge and have a mass of 1/183 ...
Flexbook - The Bohr Model of the Atom
... The development of the Bohr model is a good example of applying the scientific method. It shows how the observations of the atomic spectra led to the creation of a hypothesis about the nature of electron clouds. The hypothesis also made predictions about emissions that had not yet been observed (the ...
... The development of the Bohr model is a good example of applying the scientific method. It shows how the observations of the atomic spectra led to the creation of a hypothesis about the nature of electron clouds. The hypothesis also made predictions about emissions that had not yet been observed (the ...
Chapter 17 Thermodynamics: Directionality of Chemical Reactions
... ΔG° = -3164.6 kJ/mol Kinetically stable Product-favored, but too slow to be important. • Diamond is thermodynamically unstable Cdiamond → Cgraphite ΔG° = -2.9 kJ/mol • Kinetically stable, too slow to be important. • Ea is too large ...
... ΔG° = -3164.6 kJ/mol Kinetically stable Product-favored, but too slow to be important. • Diamond is thermodynamically unstable Cdiamond → Cgraphite ΔG° = -2.9 kJ/mol • Kinetically stable, too slow to be important. • Ea is too large ...
Mass # = Atomic # + # Neutrons
... If two or more compounds are composed of the same two elements, then the mass ratio(s) of one of the elements will always be a ratio of small whole numbers. Example: at least three different compounds containing just chromium and chlorine are known. Data for these three compounds is given in the tab ...
... If two or more compounds are composed of the same two elements, then the mass ratio(s) of one of the elements will always be a ratio of small whole numbers. Example: at least three different compounds containing just chromium and chlorine are known. Data for these three compounds is given in the tab ...
Final Review 2006
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
Stoichiometry
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
High School Chemistry Essential Questions
... 2. What observations about chemical systems and chemical interactions lead us to form the physical, graphical, and mathematical models that we use to represent, analyze, and communicate structure and relationships in chemical systems and chemical interactions? 3. How do we use the physical models, s ...
... 2. What observations about chemical systems and chemical interactions lead us to form the physical, graphical, and mathematical models that we use to represent, analyze, and communicate structure and relationships in chemical systems and chemical interactions? 3. How do we use the physical models, s ...
Chapter 1: Matter and Measurement
... radioactive isotopes of elements of low atomic number. Its percent natural abundance among K isotopes is 0.012%. How many 40K atoms do you ingest by drinking one cup of whole milk containing 371 mg of K? Want atoms of 40K, need atoms of K, Want atoms of K, need moles of K, Want moles of K, need mass ...
... radioactive isotopes of elements of low atomic number. Its percent natural abundance among K isotopes is 0.012%. How many 40K atoms do you ingest by drinking one cup of whole milk containing 371 mg of K? Want atoms of 40K, need atoms of K, Want atoms of K, need moles of K, Want moles of K, need mass ...
Electrons in Atoms
... • The arrangement of electrons in an atom is called the atom’s electron configuration. • Electron configurations are defined by the aufbau principle, the Pauli exclusion principle, and Hund’s rule. • An element’s valence electrons determine the chemical properties of the element. • Electron configur ...
... • The arrangement of electrons in an atom is called the atom’s electron configuration. • Electron configurations are defined by the aufbau principle, the Pauli exclusion principle, and Hund’s rule. • An element’s valence electrons determine the chemical properties of the element. • Electron configur ...
CHE 105 Spring 2016 Exam 3
... Select all of the true statements about quantum numbers. A. The principal quantum number, n, determines the shape of an orbital. ✓B. Energy is absorbed when an electron moves from a shell with principal quantum number n = 1 to one with n = 3. C. The angular momentum quantum number, l, determines how ...
... Select all of the true statements about quantum numbers. A. The principal quantum number, n, determines the shape of an orbital. ✓B. Energy is absorbed when an electron moves from a shell with principal quantum number n = 1 to one with n = 3. C. The angular momentum quantum number, l, determines how ...
Unit 1: Stoichiometry
... You can convert an isotope name to isotope notation using the mass number and the atomic number for the element as shown in Example 1. Example 1: Write the isotope notation for oxygen‐18 and list the number of protons, electrons, and neutrons in an atom of this isotope. Answer: Oxygen ‐ atomic numbe ...
... You can convert an isotope name to isotope notation using the mass number and the atomic number for the element as shown in Example 1. Example 1: Write the isotope notation for oxygen‐18 and list the number of protons, electrons, and neutrons in an atom of this isotope. Answer: Oxygen ‐ atomic numbe ...
Name_______________________________________________
... d. the more likely it is that the substances is polar-covalent 2. In general, intermolecular forces are a. stronger than bonds that join atoms in molecules b. weaker than bonds that join atoms in molecules, but stronger than ionic bonds c. stronger than bonds that join metal atoms in solid metals d. ...
... d. the more likely it is that the substances is polar-covalent 2. In general, intermolecular forces are a. stronger than bonds that join atoms in molecules b. weaker than bonds that join atoms in molecules, but stronger than ionic bonds c. stronger than bonds that join metal atoms in solid metals d. ...
Honors Chemistry Worksheet – Atomic Theory and the Basic Atom
... ion with a negative two charge would be deflected to a greater extent as both mass and velocity of the particles are the same, making momentum and inertia the same. However, Coulomb’s law would indicate the electrostatic force would be greater for the ion of greater charge as electrostatic force is ...
... ion with a negative two charge would be deflected to a greater extent as both mass and velocity of the particles are the same, making momentum and inertia the same. However, Coulomb’s law would indicate the electrostatic force would be greater for the ion of greater charge as electrostatic force is ...
Atoms, Molecules, and Ions
... d. The correct name of this compound in water is hydrobromic acid. e. This is an ionic compound in which the metal cation (Li+) has only one charge. The correct name is lithium carbonate. f. This is an ionic compound in which the metal cation (K) has only one charge. The correct name is potassium d ...
... d. The correct name of this compound in water is hydrobromic acid. e. This is an ionic compound in which the metal cation (Li+) has only one charge. The correct name is lithium carbonate. f. This is an ionic compound in which the metal cation (K) has only one charge. The correct name is potassium d ...
Review History of the Atom
... Dalton devised the first modern atomic model. Which one of the following characteristics is NOT part of Dalton's atomic model? a. Atoms of different elements are different. b. All atoms of the same element are identical. c. Atoms combine to form compounds. d. Atoms consist of positive particles and ...
... Dalton devised the first modern atomic model. Which one of the following characteristics is NOT part of Dalton's atomic model? a. Atoms of different elements are different. b. All atoms of the same element are identical. c. Atoms combine to form compounds. d. Atoms consist of positive particles and ...
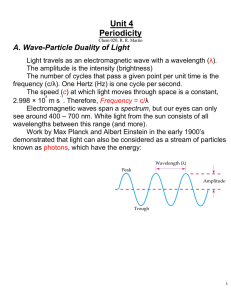
Unit 4 Periodicity
... o Of course, this requires you to know that s holds two electrons, p holds six, d holds ten, and f holds fourteen. However, this same information can be obtained from the periodic table. ...
... o Of course, this requires you to know that s holds two electrons, p holds six, d holds ten, and f holds fourteen. However, this same information can be obtained from the periodic table. ...