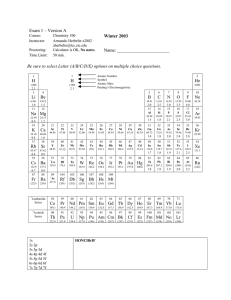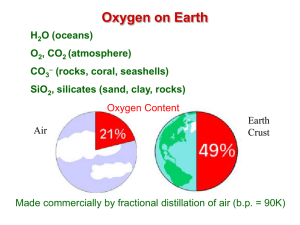
pblock - Chemistry Courses
... • It can make stable bonds with itself • It can make multiple bonds to C, N, O • The C-H bond is nonpolar, but bonds to other elements (N, O, halogens) are polar This is why life is based on the chemistry of carbon ...
... • It can make stable bonds with itself • It can make multiple bonds to C, N, O • The C-H bond is nonpolar, but bonds to other elements (N, O, halogens) are polar This is why life is based on the chemistry of carbon ...
Grade 8 th Science Curriculum Scope and Sequence
... compare the properties of compounds with those of the elements from which they are made. Provide evidence to support the fact that the idea of atoms explains conservation of matter. a. Use appropriate tools to measure temperature (thermometer), mass (balance), length (meter stick), volume (graduated ...
... compare the properties of compounds with those of the elements from which they are made. Provide evidence to support the fact that the idea of atoms explains conservation of matter. a. Use appropriate tools to measure temperature (thermometer), mass (balance), length (meter stick), volume (graduated ...
Test - Regents
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Build an Atom Scripted - UTeach Outreach
... and explain how an atom is put together and how the parts of an atom relate to one another. The most commonly used model is called the Bohr model of the atom. In the Bohr model, an atom is made up of smaller pieces that are arranged similarly to a solar system. In a solar system, the large sun is in ...
... and explain how an atom is put together and how the parts of an atom relate to one another. The most commonly used model is called the Bohr model of the atom. In the Bohr model, an atom is made up of smaller pieces that are arranged similarly to a solar system. In a solar system, the large sun is in ...
Build an Atom Scripted
... and explain how an atom is put together and how the parts of an atom relate to one another. The most commonly used model is called the Bohr model of the atom. In the Bohr model, an atom is made up of smaller pieces that are arranged similarly to a solar system. In a solar system, the large sun is in ...
... and explain how an atom is put together and how the parts of an atom relate to one another. The most commonly used model is called the Bohr model of the atom. In the Bohr model, an atom is made up of smaller pieces that are arranged similarly to a solar system. In a solar system, the large sun is in ...
SAMPLE PAPER -2 Time Allowed: 3 Hrs
... Silver metal crystallises with a face centered cubic lattice. The length of unit cell is found to be 4.077x10 -8 cm. Calculate atomic radius and density of silver.(atomic mass of Ag = 108u, NA = 6.02x10 23 mol-1) Calculate packing efficiency in fcc structure. Manu and his father went to a shop to pu ...
... Silver metal crystallises with a face centered cubic lattice. The length of unit cell is found to be 4.077x10 -8 cm. Calculate atomic radius and density of silver.(atomic mass of Ag = 108u, NA = 6.02x10 23 mol-1) Calculate packing efficiency in fcc structure. Manu and his father went to a shop to pu ...
Unit Description and Student Understandings
... Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter? Can student describe the effects of various factors on the rate of a chemical reaction? Can students describe radioactivity? Can students differentiate between atomic fission and fusion? ...
... Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter? Can student describe the effects of various factors on the rate of a chemical reaction? Can students describe radioactivity? Can students differentiate between atomic fission and fusion? ...
support material
... Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest unit that takes part in chemical combinati ...
... Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest unit that takes part in chemical combinati ...
Part II - American Chemical Society
... a. The Mg2+ ion is smaller and has a higher charge than the Mg+ ion, so the lattice energy that arises when Mg2+ ions form compounds is much greater than what would be observed if Mg+ ions formed compounds. The increase in lattice energy more than offsets the larger ionization energy of the Mg2+ ion ...
... a. The Mg2+ ion is smaller and has a higher charge than the Mg+ ion, so the lattice energy that arises when Mg2+ ions form compounds is much greater than what would be observed if Mg+ ions formed compounds. The increase in lattice energy more than offsets the larger ionization energy of the Mg2+ ion ...
Molecular Formulas - Hatboro
... number of neutrons), X is the element’s symbol, and Z is the atomic number (number of protons). This information can also be found on the periodic table. Use this information to fill in the chart for the following elements Symbol ...
... number of neutrons), X is the element’s symbol, and Z is the atomic number (number of protons). This information can also be found on the periodic table. Use this information to fill in the chart for the following elements Symbol ...
Why Study Chemistry
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) ...
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) ...
ch05.ppt - James Goodwin
... Dalton’s Model of the Atom (1803-1810) 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and size ...
... Dalton’s Model of the Atom (1803-1810) 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and size ...
Your Turn
... Dalton’s Model of the Atom (1803-1810) 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and size ...
... Dalton’s Model of the Atom (1803-1810) 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and size ...
MidtermReview2012
... 3. When a small amount of carbon is mixed in with molten iron, the cooled resulting alloy is called steel. Would you consider the iron to be changed physically or chemically? Explain. ...
... 3. When a small amount of carbon is mixed in with molten iron, the cooled resulting alloy is called steel. Would you consider the iron to be changed physically or chemically? Explain. ...
chemistry
... 29 Energy is released during the fission of Pu-239 atoms as a result of the (1) formation of covalent bonds (2) formation of ionic bonds (3) conversion of matter to energy (4) conversion of energy to matter ...
... 29 Energy is released during the fission of Pu-239 atoms as a result of the (1) formation of covalent bonds (2) formation of ionic bonds (3) conversion of matter to energy (4) conversion of energy to matter ...
FREE Sample Here
... 19) From its atomic number of 15, it is possible to predict that the phosphorus atom has A) 15 neutrons. B) 15 protons. C) 15 electrons. D) 8 electrons in its outermost electron shell. E) 15 protons and 15 electrons. Answer: E Topic: Concept 2.2 Skill: Knowledge/Comprehension ...
... 19) From its atomic number of 15, it is possible to predict that the phosphorus atom has A) 15 neutrons. B) 15 protons. C) 15 electrons. D) 8 electrons in its outermost electron shell. E) 15 protons and 15 electrons. Answer: E Topic: Concept 2.2 Skill: Knowledge/Comprehension ...
Thermochemistry
... DH0rxn = [ cDH0f (C) + dDH0f (D) ] - [ aDH0f (A) + bDH0f (B) ] 0 (reactants) DH S DH0rxn = S DH0 (products) f f ...
... DH0rxn = [ cDH0f (C) + dDH0f (D) ] - [ aDH0f (A) + bDH0f (B) ] 0 (reactants) DH S DH0rxn = S DH0 (products) f f ...
Unit 2 learning targets
... proposed a different atomic theory. Like Democritus, Dalton proposed that atoms could not be divided into smaller parts. However, unlike Democritus, Dalton performed scientific experiments to find data to support his theory. Dalton’s experiments showed that atoms of different elements could combine ...
... proposed a different atomic theory. Like Democritus, Dalton proposed that atoms could not be divided into smaller parts. However, unlike Democritus, Dalton performed scientific experiments to find data to support his theory. Dalton’s experiments showed that atoms of different elements could combine ...
Thermobest for Chem1
... DH0rxn = [ cDH0f (C) + dDH0f (D) ] - [ aDH0f (A) + bDH0f (B) ] 0 (reactants) DH S DH0rxn = S DH0 (products) f f ...
... DH0rxn = [ cDH0f (C) + dDH0f (D) ] - [ aDH0f (A) + bDH0f (B) ] 0 (reactants) DH S DH0rxn = S DH0 (products) f f ...
No Slide Title - McMaster Chemistry
... Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
... Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
Document
... The Law of Conservation of Mass says that a chemical equation must have the same number of atoms of a given kind on each side (a chemical reaction cannot create or destroy carbon, or oxygen, or hydrogen, or ...
... The Law of Conservation of Mass says that a chemical equation must have the same number of atoms of a given kind on each side (a chemical reaction cannot create or destroy carbon, or oxygen, or hydrogen, or ...
Chemistry 139
... (6 pts) A dedicated Chemistry 100 student tried to repeat the paper chromatography experiment from lecture. Based on the individual results, Label each of the following samples as ...
... (6 pts) A dedicated Chemistry 100 student tried to repeat the paper chromatography experiment from lecture. Based on the individual results, Label each of the following samples as ...