Homework,1 Atoms, molecules, and ions
... d) FeO, iron(III) oxide e) NH4+, ammonia 9- Which of these acids has a name that begins with hydro-? a) HCl b) HClO c) HClO2 d) HClO3 e) HClO4 10- Which of the following compounds is not a possible ionic compound? a) CsCl b) SrO c) GaF3 d) Cs2O e) NaF2 ...
... d) FeO, iron(III) oxide e) NH4+, ammonia 9- Which of these acids has a name that begins with hydro-? a) HCl b) HClO c) HClO2 d) HClO3 e) HClO4 10- Which of the following compounds is not a possible ionic compound? a) CsCl b) SrO c) GaF3 d) Cs2O e) NaF2 ...
Openstax - Chemistry - Answer Key
... 97. −33.4 °C, 239.8 K 99. 113 °F Chapter 2 1. The starting materials consist of one green sphere and two purple spheres. The products consist of two green spheres and two purple spheres. This violates Dalton’s postulate that that atoms are not created during a chemical change, but are merely redistr ...
... 97. −33.4 °C, 239.8 K 99. 113 °F Chapter 2 1. The starting materials consist of one green sphere and two purple spheres. The products consist of two green spheres and two purple spheres. This violates Dalton’s postulate that that atoms are not created during a chemical change, but are merely redistr ...
2004 NEACS Ashdown Exam 1. The allotrope of carbon shown to
... 66. Oxalic acid, H2C2O4, has two pKa values, 1.25 and 4.27. A 0.100 M solution of oxalic acid was titrated with a 0.100 M solution of NaOH. What is the pH at the second equivalence point is (A) 1.23 (B) 5.60 (C) 8.40 (D) 12.52 67. Which of the following compounds contains only 1 double bond? (A) CO2 ...
... 66. Oxalic acid, H2C2O4, has two pKa values, 1.25 and 4.27. A 0.100 M solution of oxalic acid was titrated with a 0.100 M solution of NaOH. What is the pH at the second equivalence point is (A) 1.23 (B) 5.60 (C) 8.40 (D) 12.52 67. Which of the following compounds contains only 1 double bond? (A) CO2 ...
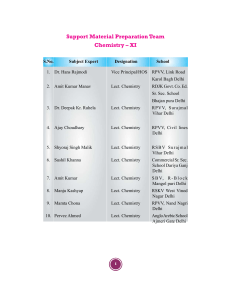
Support Material
... Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson’s model and its limitations. Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, cle Broglie’s relationship, Heisenberg unce ...
... Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson’s model and its limitations. Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, cle Broglie’s relationship, Heisenberg unce ...
Chapter 6 Electronic Structure of Atoms
... within a cluster, clusters are more or less aligned and substance acts as a magnet. Don't drop it!! •When all of the domains, represented by these arrows are aligned, it behaves as a magnet. This is what happens if you drop it! The domains go indifferent directions and it no longer operates as a mag ...
... within a cluster, clusters are more or less aligned and substance acts as a magnet. Don't drop it!! •When all of the domains, represented by these arrows are aligned, it behaves as a magnet. This is what happens if you drop it! The domains go indifferent directions and it no longer operates as a mag ...
CHEMISTRY
... In the previously studied slides, the mass of the reaction products were calculated, by assuming the complete transformation of the reagents in the corresponding products. This is not always true. Most of the reactions are of equilibrium, i.e. The transformation of the reagents into the products is ...
... In the previously studied slides, the mass of the reaction products were calculated, by assuming the complete transformation of the reagents in the corresponding products. This is not always true. Most of the reactions are of equilibrium, i.e. The transformation of the reagents into the products is ...
chemistry
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 35 Which general trend is found in Period 3 as the elements are considered in order of increasing ato ...
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 35 Which general trend is found in Period 3 as the elements are considered in order of increasing ato ...
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR
... VALENCE BOND THEORY Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of ...
... VALENCE BOND THEORY Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of ...
doc file
... Place a neon gas discharge tube into the power supply and describe and record the observed color. Examine the emitted light through the spectroscope and record in the Data the color all intense lines (V, B, G, Y, O, R). Replace the neon tube with each of the following gas discharge tubes: argon, kry ...
... Place a neon gas discharge tube into the power supply and describe and record the observed color. Examine the emitted light through the spectroscope and record in the Data the color all intense lines (V, B, G, Y, O, R). Replace the neon tube with each of the following gas discharge tubes: argon, kry ...
Spring Exam 4 - Chemistry
... order diffraction (n=1) is measured to be 42.6o. What is the distance (in pm) between the layers of atoms responsible for the diffraction? ...
... order diffraction (n=1) is measured to be 42.6o. What is the distance (in pm) between the layers of atoms responsible for the diffraction? ...
Properties and Changes of Matter
... This curve can also work in reverse if energy is being taken away. ...
... This curve can also work in reverse if energy is being taken away. ...
Unit 4 - cloudfront.net
... 3. Coal is a complex organic material with ______________ as the primary element. It also contains H, O, N, and S. Complete combustion of carbon produces__________. a. Burning coal also produces CO and soot (unburned carbon). Noncombustible matter is left unburned as ashes (fly ash). Sulfur dioxide ...
... 3. Coal is a complex organic material with ______________ as the primary element. It also contains H, O, N, and S. Complete combustion of carbon produces__________. a. Burning coal also produces CO and soot (unburned carbon). Noncombustible matter is left unburned as ashes (fly ash). Sulfur dioxide ...
FREE Sample Here
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
FREE Sample Here
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
... 39. The element carbon forms the basis of study in Organic Chemistry. Which statement about the element carbon is FALSE? A) Carbon is a period 2 element. B) Carbon is a group 4 element. C) Carbon is a nonmetal. D) Carbon atoms have six valence electrons. E) Carbon atoms have six protons. Ans: D 40. ...
Preview Sample 1
... 49) What do the four elements most abundant in life-carbon, oxygen, hydrogen, and nitrogen-have in common? A) They all have the same number of valence electrons. B) Each element exists in only one isotopic form. C) They are equal in electronegativity. D) They are elements produced only by living cel ...
... 49) What do the four elements most abundant in life-carbon, oxygen, hydrogen, and nitrogen-have in common? A) They all have the same number of valence electrons. B) Each element exists in only one isotopic form. C) They are equal in electronegativity. D) They are elements produced only by living cel ...
AGE article for Sept 2013
... able to use your rules to write half equations for acidified MnO4– ions being reduced to Mn2+, and acidified Cr2O72– ions being reduced to Cr3+. Either of these may well be relevant when considering the oxidation of an alkanol to an alkanoic acid. The half equations for a hydrogen-oxygen fuel cell a ...
... able to use your rules to write half equations for acidified MnO4– ions being reduced to Mn2+, and acidified Cr2O72– ions being reduced to Cr3+. Either of these may well be relevant when considering the oxidation of an alkanol to an alkanoic acid. The half equations for a hydrogen-oxygen fuel cell a ...
Chemistry with Physics Structure for Quiz
... is easily compressed, and mixes with any other gases. ...
... is easily compressed, and mixes with any other gases. ...
writing chemical equations
... below. The series are listed in descending order of chemical reactivity, with the most active metals and halogens at the top (the elements most likely to undergo oxidation). Any metal on the list will replace the ions of those metals (to undergo reduction) that appear anywhere underneath it on the l ...
... below. The series are listed in descending order of chemical reactivity, with the most active metals and halogens at the top (the elements most likely to undergo oxidation). Any metal on the list will replace the ions of those metals (to undergo reduction) that appear anywhere underneath it on the l ...
Final Review 2006
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
MISE - Physical Basis of Chemistry
... Up to now, we’ve been talking about relative atomic weights and we have been working in ratio - using the “triangle”. Since individual weights appear in the periodic table, there has to be a mass standard, i.e., a reference mass - so that the ratio of atomic weights can become individual values. Sin ...
... Up to now, we’ve been talking about relative atomic weights and we have been working in ratio - using the “triangle”. Since individual weights appear in the periodic table, there has to be a mass standard, i.e., a reference mass - so that the ratio of atomic weights can become individual values. Sin ...
Chemistry
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
Atomic Theory Timeline Project
... link at bottom left, then to History of Atomic Theory at top left on next ...
... link at bottom left, then to History of Atomic Theory at top left on next ...
Example
... 2. Take first reactant, calculate theoretical yield of desired product (in grams). 3. Repeat #2 for second reactant. 4. Compare results. Whichever reactant gives the LEAST amount of the product is the limiting reactant and determines the theoretical yield. The other is in excess. ...
... 2. Take first reactant, calculate theoretical yield of desired product (in grams). 3. Repeat #2 for second reactant. 4. Compare results. Whichever reactant gives the LEAST amount of the product is the limiting reactant and determines the theoretical yield. The other is in excess. ...