CHAPTER 23 THE TRANSITION ELEMENTS AND THEIR
... so the charge on the cation is +2. The anion is SO42-. Only one sulfate is needed to make a neutral salt. The formula of the compound is [Zn(NH3)4]SO4. b) The cation is pentaamminechlorochromium(III) ion. The ligands are 5 NH3 from pentaammine, and one chloride from chloro. The chromium ion has a ch ...
... so the charge on the cation is +2. The anion is SO42-. Only one sulfate is needed to make a neutral salt. The formula of the compound is [Zn(NH3)4]SO4. b) The cation is pentaamminechlorochromium(III) ion. The ligands are 5 NH3 from pentaammine, and one chloride from chloro. The chromium ion has a ch ...
Notes for Quarter I
... interference, the resulting wave has a smaller amplitude than the individual waves had. Diffracted light waves can cause both types of interference (Fig. 9, p. 650). Section 4 – Light and Color When light strikes any form of matter, it can interact with the matter in three different ways-it can be r ...
... interference, the resulting wave has a smaller amplitude than the individual waves had. Diffracted light waves can cause both types of interference (Fig. 9, p. 650). Section 4 – Light and Color When light strikes any form of matter, it can interact with the matter in three different ways-it can be r ...
Review Study Guide for the Final
... What is it called when you have more electrons than protons? ...
... What is it called when you have more electrons than protons? ...
What are atoms?
... matter. Dalton's ideas were based on many scientific experiments and observations. The ideas formed a theory that led to our modern atomic theory. You may wonder how we could know anything about a particle of matter that is too small to see and almost too small to measure. Scientists have learned ho ...
... matter. Dalton's ideas were based on many scientific experiments and observations. The ideas formed a theory that led to our modern atomic theory. You may wonder how we could know anything about a particle of matter that is too small to see and almost too small to measure. Scientists have learned ho ...
chm 434f/1206f solid state materials chemistry
... • Fundamental aspect of solid state chemistry • Chemical reactivity of solid state materials depends on form and physical dimensions as well as structure and imperfections of reactants and products • Factors governing solid state reactivity underpin concepts and methods for the synthesis of new soli ...
... • Fundamental aspect of solid state chemistry • Chemical reactivity of solid state materials depends on form and physical dimensions as well as structure and imperfections of reactants and products • Factors governing solid state reactivity underpin concepts and methods for the synthesis of new soli ...
Multiple Pathways To Success Quarter 3 Learning Module
... ● Crash Course Chemistry #24 Bonding Theories - Lewis Structure ● Crash Course Chemistry #23 Polar and Nonpolar Bonds ...
... ● Crash Course Chemistry #24 Bonding Theories - Lewis Structure ● Crash Course Chemistry #23 Polar and Nonpolar Bonds ...
Chemical Equations
... This is the resonance arrow which is used to indicate the different resonance structures of a substance. This means "does not yield" in case you need to make the point! ...
... This is the resonance arrow which is used to indicate the different resonance structures of a substance. This means "does not yield" in case you need to make the point! ...
chapter 3
... 3.4 The Chemical Mole The mole is defined as the # of C atoms in exactly 12 grams of Carbon-12: 1 mole C atoms = 6.02x1023 C atoms ...
... 3.4 The Chemical Mole The mole is defined as the # of C atoms in exactly 12 grams of Carbon-12: 1 mole C atoms = 6.02x1023 C atoms ...
THE GENERAL LAW OF CHEMICAL KINETICS, DOES IT EXIST?
... through a hole in the floor into the bell-ringer room. But let us imagine that every rope instead of putting into motion one bell participates in the motion of many parts of the mechanism and that the motion of every bell is determined not only by the motions of its own rope but the motions of sever ...
... through a hole in the floor into the bell-ringer room. But let us imagine that every rope instead of putting into motion one bell participates in the motion of many parts of the mechanism and that the motion of every bell is determined not only by the motions of its own rope but the motions of sever ...
Date - PetyaPisanScienceAQ
... Physical Differences: Magnesium is not a good conductor of electricity but copper is a great conductor of electricity and that is why they use it in cables. ...
... Physical Differences: Magnesium is not a good conductor of electricity but copper is a great conductor of electricity and that is why they use it in cables. ...
Comparing Free Energies
... This definition, together with the 2nd Law of Thermodynamics as expressed in Eq. (5.5), imply that chemical reactions will occur in the direction for which DGrxn is negative (DGrxn < 0), this is, in the direction in which the Gibbs free energy of the system decreases. The more negative the change in ...
... This definition, together with the 2nd Law of Thermodynamics as expressed in Eq. (5.5), imply that chemical reactions will occur in the direction for which DGrxn is negative (DGrxn < 0), this is, in the direction in which the Gibbs free energy of the system decreases. The more negative the change in ...
Chemistry A level transition - baseline assessment
... Chemistry topic 1 – Electronic structure, how electrons are arranged around the nucleus A periodic table can give you the proton / atomic number of an element, this also tells you how many electrons are in the atom. You will have used the rule of electrons shell filling, where: The first shell holds ...
... Chemistry topic 1 – Electronic structure, how electrons are arranged around the nucleus A periodic table can give you the proton / atomic number of an element, this also tells you how many electrons are in the atom. You will have used the rule of electrons shell filling, where: The first shell holds ...
g moles molarity
... What happens when a solution of Na2CO3 is mixed with a solution of CaCl2? First assume everbody goes into solution or already in solution Identify who is charge dense and who is not charge dense ...
... What happens when a solution of Na2CO3 is mixed with a solution of CaCl2? First assume everbody goes into solution or already in solution Identify who is charge dense and who is not charge dense ...
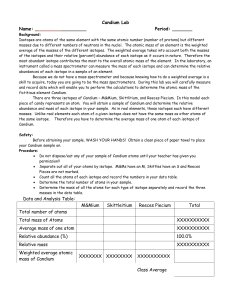
Candium Lab - OCPS TeacherPress
... 1.) Write a formula for each of the steps listed in calculations section. Example: Avg. mass of one atom = total mass of all atoms of an isotope total number of atoms of that isotope Relative Abundance = ...
... 1.) Write a formula for each of the steps listed in calculations section. Example: Avg. mass of one atom = total mass of all atoms of an isotope total number of atoms of that isotope Relative Abundance = ...
g) Chemistry 30 - Mr. Jones LHS Science
... beaker itself, what is the specific heat of the alloy? ...
... beaker itself, what is the specific heat of the alloy? ...
Chapter 6 Thermochemistry
... • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ Tro, Chemistry: A Molecular ...
... • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ Tro, Chemistry: A Molecular ...
Chapter 6 Thermochemistry
... • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ Tro, Chemistry: A Molecular ...
... • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ Tro, Chemistry: A Molecular ...
Ch. 20 - Chemical Bonds - Study Guide
... ____ 13. The chemical formula for an ionic compound of sodium and oxygen is a. NaO2. c. Na2O. b. NaO. d. Na2O2. ____ 14. The elements that make up a compound and the exact number of atoms of each element in a unit of the compound can be shown in a ____. a. subscript c. chemical formula b. chemical s ...
... ____ 13. The chemical formula for an ionic compound of sodium and oxygen is a. NaO2. c. Na2O. b. NaO. d. Na2O2. ____ 14. The elements that make up a compound and the exact number of atoms of each element in a unit of the compound can be shown in a ____. a. subscript c. chemical formula b. chemical s ...
Practice Test 2
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
Lecture 14
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
... 1. Write the correct symbols and formulas for all of the reactants and products. 2. Count the number of each type of atom on BOTH sides of the equation. 3. Insert coefficients until there are the equal numbers of each kind of atom on both sides of the equation. ...
Chapter 6 Thermochemistry
... current observations suggest that the average global air temperature has risen 0.6°C in the past 100 yrs. atmospheric models suggest that the warming effect could worsen if CO2 levels are not curbed some models predict that the result will be more severe storms, more floods and droughts, shifts in a ...
... current observations suggest that the average global air temperature has risen 0.6°C in the past 100 yrs. atmospheric models suggest that the warming effect could worsen if CO2 levels are not curbed some models predict that the result will be more severe storms, more floods and droughts, shifts in a ...
Please do not remove this page. The periodic table, constants, and
... ScanTron sheet for the following information: your name Test Form A the 9-digit ID number given above (rightmost digit blank) You should answer questions for Part II (1 - 15) on the Scantron sheet. You will not have the Scantron returned to you, so if you would like to know what you answered after t ...
... ScanTron sheet for the following information: your name Test Form A the 9-digit ID number given above (rightmost digit blank) You should answer questions for Part II (1 - 15) on the Scantron sheet. You will not have the Scantron returned to you, so if you would like to know what you answered after t ...