Figure 2 - Universität Düsseldorf
... Predictions show that the competition of exponential growth and limited natural resources can pose serious problems to our future society, affecting people’s daily life possibly already in the middle of the 21st century (Randers 2012). With the over usage of our planet´s resources, humanity will not ...
... Predictions show that the competition of exponential growth and limited natural resources can pose serious problems to our future society, affecting people’s daily life possibly already in the middle of the 21st century (Randers 2012). With the over usage of our planet´s resources, humanity will not ...
Part 2-ICHO-26-30
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
Week 1 -- Schedule
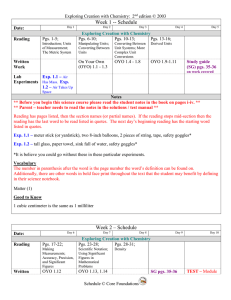
... Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has the last word to be read listed in quotes. The next day’s beginning reading has the starting word listed in quotes. Exp. 1.1 – meter stick (or yardstick), two 8-inch balloons, 2 ...
... Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has the last word to be read listed in quotes. The next day’s beginning reading has the starting word listed in quotes. Exp. 1.1 – meter stick (or yardstick), two 8-inch balloons, 2 ...
Novel Methods and Materials in Development of Liquid Carrier
... within the frame of PhD studies in the industry. Therefore, I was looking for an advisor, who would take over the supervision of my work. I led quite long e-mail correspondence with Prof. Melin whom I knew from Bayer AG, where I did my first industrial working experience in summer 1996. Meanwhile, I ...
... within the frame of PhD studies in the industry. Therefore, I was looking for an advisor, who would take over the supervision of my work. I led quite long e-mail correspondence with Prof. Melin whom I knew from Bayer AG, where I did my first industrial working experience in summer 1996. Meanwhile, I ...
CHAPTER 9
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... the Greek term for rose. It is one of the least abundant metals in the earth’s crust and was discovered by William Hyde Wollaston (1803-04) in crude platinum ore from South America. Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material ...
... the Greek term for rose. It is one of the least abundant metals in the earth’s crust and was discovered by William Hyde Wollaston (1803-04) in crude platinum ore from South America. Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material ...
THE ADSORPTION OF CO, N2 AND Li ON Ru(109) AND Ru(001
... respectively, are observed after thermal ordering of the adlayer. Nitrogen adsorbed on the iii ...
... respectively, are observed after thermal ordering of the adlayer. Nitrogen adsorbed on the iii ...
Stoichiometric Calculations
... 3 Ag(s) + 4 HNO3(aq) à 3 AgNO3(aq) + 2 H2O(l) + NO(g) A. How many moles of silver are needed to react with 40 moles of nitric acid? B. From the amount of nitric acid given in Part A, how many moles of silver nitrate will be produced? C. From the amount of nitric acid given in P ...
... 3 Ag(s) + 4 HNO3(aq) à 3 AgNO3(aq) + 2 H2O(l) + NO(g) A. How many moles of silver are needed to react with 40 moles of nitric acid? B. From the amount of nitric acid given in Part A, how many moles of silver nitrate will be produced? C. From the amount of nitric acid given in P ...
LABORATORY MANUAL GENERAL CHEMISTRY 120 Dr. Steven Fawl
... calculation. Calculations must be neat and easy to follow; units must be used every time they apply. You may add paper to your report, if you need additional room for your calculations. (Hint: do your calculations on scratch paper first, and then copy to report.) It is very important that you do no ...
... calculation. Calculations must be neat and easy to follow; units must be used every time they apply. You may add paper to your report, if you need additional room for your calculations. (Hint: do your calculations on scratch paper first, and then copy to report.) It is very important that you do no ...
006 Thermochemistry
... 54. 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C. If the following reaction occurs, then what temperature will the water reach, assuming that the cup is a perfect insulator and that the cup absorbs only a negligible amount of heat? [specific heat of water = 4.18 ...
... 54. 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C. If the following reaction occurs, then what temperature will the water reach, assuming that the cup is a perfect insulator and that the cup absorbs only a negligible amount of heat? [specific heat of water = 4.18 ...
AQA Science GCSE Chemistry
... Checked by examiners Approved by AQA Continued success, inspiring all abilities ... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners pr ...
... Checked by examiners Approved by AQA Continued success, inspiring all abilities ... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners pr ...
HW 19
... Thus iron(III) should oxidize iodide ion to iodine. This makes the iodide ion/iodine half-reaction the anode. The standard emf can be found using Equation (19.1). D D D Ecell = Ecathode − Eanode = 0.77 V − 0.53 V = 0.24 V ...
... Thus iron(III) should oxidize iodide ion to iodine. This makes the iodide ion/iodine half-reaction the anode. The standard emf can be found using Equation (19.1). D D D Ecell = Ecathode − Eanode = 0.77 V − 0.53 V = 0.24 V ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... master. UMI films the original text directlyfrom the copy submitted. Thus, some dissertation copies are in typewriter face, while others may be from a computer printer. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also ...
... master. UMI films the original text directlyfrom the copy submitted. Thus, some dissertation copies are in typewriter face, while others may be from a computer printer. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also ...
Supplemental Problems
... All rights reserved. Permission is granted to reproduce the material contained herein on the condition that such material be reproduced only for classroom use; be provided to students, teachers, and families without charge; and be used solely in conjunction with the Chemistry: Matter and Change prog ...
... All rights reserved. Permission is granted to reproduce the material contained herein on the condition that such material be reproduced only for classroom use; be provided to students, teachers, and families without charge; and be used solely in conjunction with the Chemistry: Matter and Change prog ...
ANNEX (Manuscrits posteriors a la Comissió de Doctorat de Juliol del...
... extracted with acidic water and diethyl ether. After chromatography on silica with AcOEt, four different bands were separated. Two of these accounted for more than 90% of the collected masses, and have been the ones studied. These bands correspond to Cs[8,8’-(CH3)2-3,3’-Co(1,2-C2B9H10)2], Cs[3], and ...
... extracted with acidic water and diethyl ether. After chromatography on silica with AcOEt, four different bands were separated. Two of these accounted for more than 90% of the collected masses, and have been the ones studied. These bands correspond to Cs[8,8’-(CH3)2-3,3’-Co(1,2-C2B9H10)2], Cs[3], and ...
Chemical Quantities
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
X Science Practice Paper - Brilliant Public School Sitamarhi
... Q 1 Make two separate lists from the given data:-List A comprising of physical changes and List B comprising of chemical changes. Conversion of water in ice, burning of paper, breaking of glass bottle, rusting of iron, digestion of food ,falling of leaves from tree, melting of wax candle, Souring of ...
... Q 1 Make two separate lists from the given data:-List A comprising of physical changes and List B comprising of chemical changes. Conversion of water in ice, burning of paper, breaking of glass bottle, rusting of iron, digestion of food ,falling of leaves from tree, melting of wax candle, Souring of ...
From Kinetics to Equilibrium
... The kinetic molecular theory (KMT) explains many observations and events in chemistry. For example, gas particles move randomly in all directions, following a straight line path. Picture inflating a basketball. As you add more and more air to it, more air particles randomly collide with the inside w ...
... The kinetic molecular theory (KMT) explains many observations and events in chemistry. For example, gas particles move randomly in all directions, following a straight line path. Picture inflating a basketball. As you add more and more air to it, more air particles randomly collide with the inside w ...
Heterogeneous Catalysis and Solid Catalysts
... Catalysis is a phenomenon by which chemical reactions are accelerated by small quantities of foreign substances, called catalysts. A suitable catalyst can enhance the rate of a thermodynamically feasible reaction but cannot change the position of the thermodynamic equilibrium. Most catalysts are sol ...
... Catalysis is a phenomenon by which chemical reactions are accelerated by small quantities of foreign substances, called catalysts. A suitable catalyst can enhance the rate of a thermodynamically feasible reaction but cannot change the position of the thermodynamic equilibrium. Most catalysts are sol ...
Chapter 1 - Solutions
... The theoretical maximum amount of products formed in a chemical reaction is determined by the number of moles of the limiting reactant, along with the stoichiometry of the reaction. In a reaction with a single reactant (such as a decomposition reaction, like that in problem 80) the reaction stops wh ...
... The theoretical maximum amount of products formed in a chemical reaction is determined by the number of moles of the limiting reactant, along with the stoichiometry of the reaction. In a reaction with a single reactant (such as a decomposition reaction, like that in problem 80) the reaction stops wh ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Q3- What is a chemical equation? What are its essential features? (L-2) Ans. the qualitative and quantitative representation of a chemical reaction in short form in terms of symbols and formulae is called chemical equation. For example, on heating calcium carbonate, it gives Caco3 →Ca0 + CO2 Essenti ...
... Q3- What is a chemical equation? What are its essential features? (L-2) Ans. the qualitative and quantitative representation of a chemical reaction in short form in terms of symbols and formulae is called chemical equation. For example, on heating calcium carbonate, it gives Caco3 →Ca0 + CO2 Essenti ...
Chemistry - A Quantitative Science
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
Organic Chemistry with a Biological Emphasis Volume I
... Interestingly, birds also have a heat receptor protein which is very similar to the TrpV1 receptor in mammals, but birds are not at all sensitive to capsaicin. There is an evolutionary logic to this: it is to the pepper's advantage to be eaten by a bird rather than a mammal, because a bird can sprea ...
... Interestingly, birds also have a heat receptor protein which is very similar to the TrpV1 receptor in mammals, but birds are not at all sensitive to capsaicin. There is an evolutionary logic to this: it is to the pepper's advantage to be eaten by a bird rather than a mammal, because a bird can sprea ...