1999 U. S. NATIONAL CHEMISTRY OLYMPIAD
... the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 26, 1999, after which tests can be returned to students and their teachers for further study. Allow time for the student to read the directions, ask questions ...
... the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 26, 1999, after which tests can be returned to students and their teachers for further study. Allow time for the student to read the directions, ask questions ...
EVS - RSC - Developments in Microwave Chemistry
... such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic, coordination, intercalation compounds, and nanoparticles. Microwave technology als ...
... such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic, coordination, intercalation compounds, and nanoparticles. Microwave technology als ...
Chem 12 SM Ch5 Review final new ok revised
... and potential energy, as well as concepts and real world examples of each type of energy. 33. Answers may vary. Students’ diagrams should include what happens to the water molecules and any energy transfers that take place. Ideas could include: The process of liquid water turning into steam is an en ...
... and potential energy, as well as concepts and real world examples of each type of energy. 33. Answers may vary. Students’ diagrams should include what happens to the water molecules and any energy transfers that take place. Ideas could include: The process of liquid water turning into steam is an en ...
Shriver 5e Answers to Self Tests and Exercises
... removed with gradually increasing values. Removing the fifth electron requires a large increase in energy, indicating breaking into a complete subshell. S1.10 Adding another electron to C would result in ...
... removed with gradually increasing values. Removing the fifth electron requires a large increase in energy, indicating breaking into a complete subshell. S1.10 Adding another electron to C would result in ...
4. Solution Guide to Supplementary Exercises
... 50 C (1) Using new and used zinc-carbon cells at the same time in an electrical appliance is NOT dangerous, but this gives poorer results. (2) Charging a lithium ion secondary cell with a high current may overheat the pack and lead to explosion. 51 C In a magnesium-copper chemical cell, electrons fl ...
... 50 C (1) Using new and used zinc-carbon cells at the same time in an electrical appliance is NOT dangerous, but this gives poorer results. (2) Charging a lithium ion secondary cell with a high current may overheat the pack and lead to explosion. 51 C In a magnesium-copper chemical cell, electrons fl ...
Transition Metal-Modified Zirconium Phosphate Electrocatalysts for
... precious metals [15,16]. Significant research has been conducted to improve electrocatalysts through based on precious metals [15,16]. Significant research has been conducted to improve electrocatalysts a number of general strategies, including increasing the number of active sites, increasing the ...
... precious metals [15,16]. Significant research has been conducted to improve electrocatalysts through based on precious metals [15,16]. Significant research has been conducted to improve electrocatalysts a number of general strategies, including increasing the number of active sites, increasing the ...
Stoichiometry
... The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calculate the amount of product formed when given the amounts of all the reactants present. The percent yield of a ...
... The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calculate the amount of product formed when given the amounts of all the reactants present. The percent yield of a ...
Stoichiometry of Formulas and Equations
... an analogy for atoms. Suppose you have large groups of red marbles and yellow marbles; each red marble weighs 7 g and each yellow marble weighs 4 g. Right away you know that there are 12 marbles in 84 g of red marbles or in 48 g of yellow marbles. Moreover, because one red marble weighs 47 as much a ...
... an analogy for atoms. Suppose you have large groups of red marbles and yellow marbles; each red marble weighs 7 g and each yellow marble weighs 4 g. Right away you know that there are 12 marbles in 84 g of red marbles or in 48 g of yellow marbles. Moreover, because one red marble weighs 47 as much a ...
study guide spring 2012
... What is the name of a list of elements arranged according to the ease with which they undergo certain chemical reactions? a. reactivity list c. activity series b. reaction sequence d. periodic list An element in the activity series can replace any element a. in the periodic table. c. above it on the ...
... What is the name of a list of elements arranged according to the ease with which they undergo certain chemical reactions? a. reactivity list c. activity series b. reaction sequence d. periodic list An element in the activity series can replace any element a. in the periodic table. c. above it on the ...
File
... Write your name, Centre number and candidate number on the Answer Sheet in the spaces provided unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C and D. Choose the one you consider correct and re ...
... Write your name, Centre number and candidate number on the Answer Sheet in the spaces provided unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C and D. Choose the one you consider correct and re ...
Stoichiometry - Normal Community High School Chemistry
... The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calculate the amount of product formed when given the amounts of all the reactants present. The percent yield of a ...
... The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calculate the amount of product formed when given the amounts of all the reactants present. The percent yield of a ...
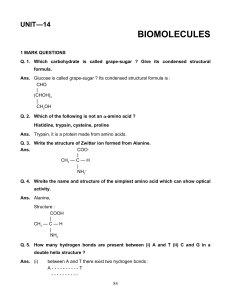
HOTS Worksheet
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
CHAPTER 12 | The Chemistry of Solids
... Some metallic bonds are quite strong as evidenced by the melting points of some metals. Whereas Na has a melting point of 97.72˚C, tungsten melts at 3422˚C. 12.23. Collect and Organize We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might a ...
... Some metallic bonds are quite strong as evidenced by the melting points of some metals. Whereas Na has a melting point of 97.72˚C, tungsten melts at 3422˚C. 12.23. Collect and Organize We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might a ...
Fundamentals of Environmental Chemistry
... rudimentary knowledge of it. There are many reasons that this is so. For example, “chemophobia,” an unreasoned fear of insidious contamination of food, water, and air with chemicals at undetectable levels that may cause cancer and other maladies is widespread among the general population. The langua ...
... rudimentary knowledge of it. There are many reasons that this is so. For example, “chemophobia,” an unreasoned fear of insidious contamination of food, water, and air with chemicals at undetectable levels that may cause cancer and other maladies is widespread among the general population. The langua ...
master ap chemistry - NelnetSolutions.com
... Peterson’s (www.petersons.com) is a leading provider of education information and advice, with books and online resources focusing on education search, test preparation, and financial aid. Its Web site offers searchable databases and interactive tools for contacting educational institutions, online ...
... Peterson’s (www.petersons.com) is a leading provider of education information and advice, with books and online resources focusing on education search, test preparation, and financial aid. Its Web site offers searchable databases and interactive tools for contacting educational institutions, online ...
Stoichiometric Calculations
... 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount of product you can get, also called the maximum yield. There are a variety of methods to determine w ...
... 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount of product you can get, also called the maximum yield. There are a variety of methods to determine w ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... century began to understand the laws of nature by observing, measuring, and performing experiments on the world around them. However, this scientific method was incorporated into chemistry slowly. Though early chemists experimented extensively, most disregarded the importance of measurement, an over ...
... century began to understand the laws of nature by observing, measuring, and performing experiments on the world around them. However, this scientific method was incorporated into chemistry slowly. Though early chemists experimented extensively, most disregarded the importance of measurement, an over ...
department of pure and applied chemistry
... GES 1012: English and Communication Skills II (2 credit units) This is a continuation of GES 1011 (English and Communication Skills 1) that introduced students to the rudiments of English for academic purposes. The focus of this course is academic writing and information literacy skills. Broadly, th ...
... GES 1012: English and Communication Skills II (2 credit units) This is a continuation of GES 1011 (English and Communication Skills 1) that introduced students to the rudiments of English for academic purposes. The focus of this course is academic writing and information literacy skills. Broadly, th ...
Physical Sciences Grade 10 Term 2
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
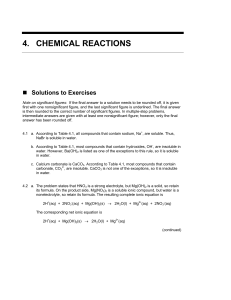
4. chemical reactions
... Note on significant figures: If the final answer to a solution needs to be rounded off, it is given first with one nonsignificant figure, and the last significant figure is underlined. The final answer is then rounded to the correct number of significant figures. In multiple-step problems, intermedi ...
... Note on significant figures: If the final answer to a solution needs to be rounded off, it is given first with one nonsignificant figure, and the last significant figure is underlined. The final answer is then rounded to the correct number of significant figures. In multiple-step problems, intermedi ...
PART 6-ICHO-26-30
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
Atmospheric evolution in the Precambrian: Constraints from water
... The effects of Po2 on mineral dissolution have been studied for Fe(II)-bearing silicate minerals (Murakami et al., 2004; Sugimori et al., 2009, 2012). Although the observation of redox-insensitive elements (e.g., Mg and Si) released during dissolution of the minerals has revealed that dissolution ra ...
... The effects of Po2 on mineral dissolution have been studied for Fe(II)-bearing silicate minerals (Murakami et al., 2004; Sugimori et al., 2009, 2012). Although the observation of redox-insensitive elements (e.g., Mg and Si) released during dissolution of the minerals has revealed that dissolution ra ...