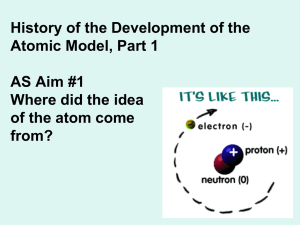
Review Packet
... After much observation and questioning, Democritus concluded that matter could not be divided into smaller and smaller pieces forever. Eventually the smallest possible piece would be obtained. All elements are composed of atoms. Atoms are indivisible and indestructible particles. Atoms of the sam ...
... After much observation and questioning, Democritus concluded that matter could not be divided into smaller and smaller pieces forever. Eventually the smallest possible piece would be obtained. All elements are composed of atoms. Atoms are indivisible and indestructible particles. Atoms of the sam ...
Chapter 5 Atomic Structure and Periodic Table 2014
... 3. Atoms of different elements can physically mix together or can chemically combine with one another in simple whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms of one element, however, are never changed into atoms of another el ...
... 3. Atoms of different elements can physically mix together or can chemically combine with one another in simple whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms of one element, however, are never changed into atoms of another el ...
early_Atomic Theory notes_academic - wths
... abundance of each isotope by its atomic mass and summing these products. Because 75.78% = 0.7578 and 24.22% = 0.2422 we have Average atomic mass = (0.7578)(34.969 amu) + (0.2422)(36.966 amu) = 26.50 amu + 8.953 amu = 35.45 amu ...
... abundance of each isotope by its atomic mass and summing these products. Because 75.78% = 0.7578 and 24.22% = 0.2422 we have Average atomic mass = (0.7578)(34.969 amu) + (0.2422)(36.966 amu) = 26.50 amu + 8.953 amu = 35.45 amu ...
Unit 3 Chap. 3 Atoms: The Building Blocks of Matter
... Electrons are held in the electron cloud by electrical attraction. ...
... Electrons are held in the electron cloud by electrical attraction. ...
The Meaning of the Wave Function
... To explain the properties of atoms we need to understand how electrons are arranged in the orbitals the wave function gives us. It is the arrangements of the electrons that determine the chemical properties of an atom. There are three rules that govern the way electrons fill orbitals: The Pauli excl ...
... To explain the properties of atoms we need to understand how electrons are arranged in the orbitals the wave function gives us. It is the arrangements of the electrons that determine the chemical properties of an atom. There are three rules that govern the way electrons fill orbitals: The Pauli excl ...
atom`s - Hauppauge School District
... • Problem #2 – Mr. Foley’s good twin decides in his class that the weighting will be quite different. For the second quarter, exams will be 50% of your grade, HW will be 30%, and Labs will be 20%. If you still score a 60 avg on exams, an 80 avg on homework, and a 100 avg on labs, do you pass? ...
... • Problem #2 – Mr. Foley’s good twin decides in his class that the weighting will be quite different. For the second quarter, exams will be 50% of your grade, HW will be 30%, and Labs will be 20%. If you still score a 60 avg on exams, an 80 avg on homework, and a 100 avg on labs, do you pass? ...
Study Guide - Flagler County Schools
... chemical energy into heat energy; chemical energy into light energy; mechanical energy to thermal energy) Know how the formula for power relates to work and time. Identify how temperature relates to k ...
... chemical energy into heat energy; chemical energy into light energy; mechanical energy to thermal energy) Know how the formula for power relates to work and time. Identify how temperature relates to k ...
Collision Theory
... 6. A student learns that ionic compounds have significant covalent character when a cation has a polarizing effect on a large anion. As a result, the student hypothesizes that salts composed of small cations and large anions should have relatively low melting points. (a) Select two compounds from th ...
... 6. A student learns that ionic compounds have significant covalent character when a cation has a polarizing effect on a large anion. As a result, the student hypothesizes that salts composed of small cations and large anions should have relatively low melting points. (a) Select two compounds from th ...
E hf + φ = mc E - No Brain Too Small
... The Bohr Model of the Atom Niels Bohr sought to refine both Rutherford and Rydberg theories by suggesting that energy travels only in distinct quanta. He developed an atomic theory that accounts for why electrons do not collapse into nuclei and why there are only particular frequencies for visible l ...
... The Bohr Model of the Atom Niels Bohr sought to refine both Rutherford and Rydberg theories by suggesting that energy travels only in distinct quanta. He developed an atomic theory that accounts for why electrons do not collapse into nuclei and why there are only particular frequencies for visible l ...
Ch 30 Nuclear Physics
... from radioactive decay. Therefore if any lead-204 is present we know that the other three lead isotopes are also present and we know their ratios. ...
... from radioactive decay. Therefore if any lead-204 is present we know that the other three lead isotopes are also present and we know their ratios. ...
atomic number
... 3) Atoms are indivisible (can’t be broken down into smaller parts) 4) Different atoms combine to make compounds 5) Atoms are rearranged during chemical changes ...
... 3) Atoms are indivisible (can’t be broken down into smaller parts) 4) Different atoms combine to make compounds 5) Atoms are rearranged during chemical changes ...
9.6
... Atomic Radius Across a Period Atomic radius decreases • Going from left to right across a period. • As more protons increase nuclear attraction for valence electrons. ...
... Atomic Radius Across a Period Atomic radius decreases • Going from left to right across a period. • As more protons increase nuclear attraction for valence electrons. ...
C2 Chemistry - Burton Borough School
... Calculating the Empirical Formula 1) Use the same table and method given for calculating reacting masses but remove the ratio row. The question will either provide the grams of each element or the percentage. Assume percentages are the same figure in grams. e.g. 12% = 12g ...
... Calculating the Empirical Formula 1) Use the same table and method given for calculating reacting masses but remove the ratio row. The question will either provide the grams of each element or the percentage. Assume percentages are the same figure in grams. e.g. 12% = 12g ...
Chapter 2 Chemical Basis of Life
... pertaining to or involving electricity (from the Greek electronamber) equal (from the Latin aequus- equal) that which produces (from the Greek genes- born or produced) balance (from the Latin libra- balance) dissolution; breaking (from the Greek lysis- dissolution) neutral; having no charge or affil ...
... pertaining to or involving electricity (from the Greek electronamber) equal (from the Latin aequus- equal) that which produces (from the Greek genes- born or produced) balance (from the Latin libra- balance) dissolution; breaking (from the Greek lysis- dissolution) neutral; having no charge or affil ...
Structure of the Atom
... vary. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple wholenumber ratios to form chemical compounds. In chemical reactions, atoms are combined, separated, or rearranged. ...
... vary. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple wholenumber ratios to form chemical compounds. In chemical reactions, atoms are combined, separated, or rearranged. ...
1 - Mr. MacGillivray
... C. Positively charged and has a low density D. Positively charged and has a high density 27. Dalton theorized that atoms are indivisible and that all atoms of an element are identical. we now know that: A. Dalton's theories are completely correct B. Atoms of an element can have different number ...
... C. Positively charged and has a low density D. Positively charged and has a high density 27. Dalton theorized that atoms are indivisible and that all atoms of an element are identical. we now know that: A. Dalton's theories are completely correct B. Atoms of an element can have different number ...
Re-typed from The Ultimate Chemical Equations Handbook by
... Re-typed from The Ultimate Chemical Equations Handbook by Hague and Smith Ternary Nomenclature: Acids and salts Containing Halogens and/or Oxygen 1. The halogens, with their variable oxidation numbers, allow for a great variety of compounds. 2. A good way to learn ternary nomenclature is to start ...
... Re-typed from The Ultimate Chemical Equations Handbook by Hague and Smith Ternary Nomenclature: Acids and salts Containing Halogens and/or Oxygen 1. The halogens, with their variable oxidation numbers, allow for a great variety of compounds. 2. A good way to learn ternary nomenclature is to start ...
Unit C3, C3.1
... The periodic table on the Data Sheet may help you to answer some of these questions. (a) ...
... The periodic table on the Data Sheet may help you to answer some of these questions. (a) ...
UNIT 2 – THE ATOM - Neshaminy School District
... Write the symbol of the element. If the atom has a charge, it must be written with a positive or a negative and the number of the charge as a superscript behind the symbol. If there is no charge on the atom, then just write the symbol. ...
... Write the symbol of the element. If the atom has a charge, it must be written with a positive or a negative and the number of the charge as a superscript behind the symbol. If there is no charge on the atom, then just write the symbol. ...
Chapter 4 Chem classnotes
... The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. Quantum theory describes mathematically the wave properties of electrons and other very small particles. An orbital is a three dimensiona ...
... The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. Quantum theory describes mathematically the wave properties of electrons and other very small particles. An orbital is a three dimensiona ...
Atomic Theory Powerpoint
... • The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable level, or shell, by absorbing energy. This higher-energy, lessstable state is called the electron’s excited state. • After it’s done being excited, the electron can return to ...
... • The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable level, or shell, by absorbing energy. This higher-energy, lessstable state is called the electron’s excited state. • After it’s done being excited, the electron can return to ...