Unit A Remediation Review
... 11. What is the difference between a physical change and a chemical change? Give an example of each. 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) ...
... 11. What is the difference between a physical change and a chemical change? Give an example of each. 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) ...
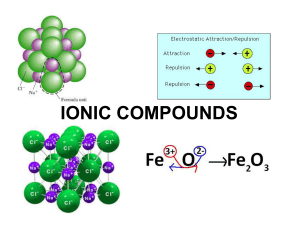
Section 8.3 Names and Formulas of Ionic Compounds Formula Unit
... (written as a roman numeral in parentheses after the name of the cation). 5. If the compound contains a polyatomic ion, simply name the ion. ...
... (written as a roman numeral in parentheses after the name of the cation). 5. If the compound contains a polyatomic ion, simply name the ion. ...
Chemistry Mid-Term Review: 2015-2016
... 5. What distinguishes the atoms of one element from the atoms of another? 6. What equation tells you how to calculate the number of neutrons in an atom? 7. What is the charge- positive or negative, of the nucleus of every atom? 8. Why is an atom electrically neutral? 9. What does the atomic number o ...
... 5. What distinguishes the atoms of one element from the atoms of another? 6. What equation tells you how to calculate the number of neutrons in an atom? 7. What is the charge- positive or negative, of the nucleus of every atom? 8. Why is an atom electrically neutral? 9. What does the atomic number o ...
What are the general types of reactions?
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
EPSc 446 STABLE ISOTOPE GEOCHEMISTRY Instructor: Bob Criss
... Found that a few α particles were deflected through large angles- up to 180°. ...
... Found that a few α particles were deflected through large angles- up to 180°. ...
File
... In 1917 Rutherford bombarded nitrogen gas with alpha particles and observed hydrogen nuclei being emitted from the gas (Rutherford recognized these, because he had previously obtained them bombarding hydrogen with alpha particles, and observing hydrogen nuclei in the products). Rutherford concluded ...
... In 1917 Rutherford bombarded nitrogen gas with alpha particles and observed hydrogen nuclei being emitted from the gas (Rutherford recognized these, because he had previously obtained them bombarding hydrogen with alpha particles, and observing hydrogen nuclei in the products). Rutherford concluded ...
Periodic Table: Why the repetition
... • As Z increases, what if new electron is placed in same shell? • As Z increases, what if each electron is placed further away from nucleus? • Any other possibilities to be considered? ...
... • As Z increases, what if new electron is placed in same shell? • As Z increases, what if each electron is placed further away from nucleus? • Any other possibilities to be considered? ...
Average Atomic Mass notes
... – We have different isotopes of the same atom • They are the same type of atom but they have a different mass because they have a different number of neutrons ...
... – We have different isotopes of the same atom • They are the same type of atom but they have a different mass because they have a different number of neutrons ...
Is the Bohr`s quantization hypothesis necessary?
... that its angular momentum could only have certain discrete values, these being integer multiples of a certain basic value [6]. This was his “ad hoc” assumption, introduced by hand into the theory. In 1913, therefore, he proposed the following for the atomic model [7]: 1. The atom would be composed o ...
... that its angular momentum could only have certain discrete values, these being integer multiples of a certain basic value [6]. This was his “ad hoc” assumption, introduced by hand into the theory. In 1913, therefore, he proposed the following for the atomic model [7]: 1. The atom would be composed o ...
Chemistry Notes
... Catalysts are used to make very difficult reactions happen. They help very large molecules combine. If you look at the graph, you will notice that when the activation energy is lower, the products can combine easier. So the forward and reverse reactions are both speeded up. ...
... Catalysts are used to make very difficult reactions happen. They help very large molecules combine. If you look at the graph, you will notice that when the activation energy is lower, the products can combine easier. So the forward and reverse reactions are both speeded up. ...
GHW - Louisiana Tech University
... Of course if we used some other mass unit for the mole such as "pound mole", the "number" would be different than 6.022 x 1023. 21) Given 5 moles of Sulfuric Acid having a formula of H2SO4 answer the following questions: ...
... Of course if we used some other mass unit for the mole such as "pound mole", the "number" would be different than 6.022 x 1023. 21) Given 5 moles of Sulfuric Acid having a formula of H2SO4 answer the following questions: ...
Atoms Notes Matter: anything that has mass and takes up space 3
... Matter: ______________________________________________________________________ ...
... Matter: ______________________________________________________________________ ...
Atomic Number
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
ionic bond. - cloudfront.net
... • Alloys -metallic substances composed of two or more elements; at least one of these elements is a metal. • Alloys are important because the properties of an alloy are often superior to those of its component elements. ...
... • Alloys -metallic substances composed of two or more elements; at least one of these elements is a metal. • Alloys are important because the properties of an alloy are often superior to those of its component elements. ...
Chem 1411 Chapter 4
... An oxidation reaction is the one that involves loss of electrons and a reduction reaction involves the gain of electrons. A reaction in which oxidation and reduction occurs simultaneously is called a redox reaction. The species that undergoes oxidation is the reductant (reducing agent); and the spec ...
... An oxidation reaction is the one that involves loss of electrons and a reduction reaction involves the gain of electrons. A reaction in which oxidation and reduction occurs simultaneously is called a redox reaction. The species that undergoes oxidation is the reductant (reducing agent); and the spec ...
elements and isotopes - vocabulary
... The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exactly 12 amu. naturally occurring isotope An isotope ...
... The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exactly 12 amu. naturally occurring isotope An isotope ...
2 (aq)
... • Is a chemical change in which an element or a compound reacts with oxygen often producing energy of the form of heat and light – Examples: 2C8H16(l) + 25O2(g) 16CO2(g) + 18H2O(l) 2Mg(s) + O2(g) 2MgO(s) S(s) + O2(g) SO2(g) ...
... • Is a chemical change in which an element or a compound reacts with oxygen often producing energy of the form of heat and light – Examples: 2C8H16(l) + 25O2(g) 16CO2(g) + 18H2O(l) 2Mg(s) + O2(g) 2MgO(s) S(s) + O2(g) SO2(g) ...
Atomic Mass
... 1932 – confirmed the existence of the neutron – a particle with no charge, but a mass nearly equal to a proton He was trying to identify the extra mass of an atomic nucleus by firing alpha particles into a beryllium target and allowing the resulting radiation to interact with paraffin wax. The ...
... 1932 – confirmed the existence of the neutron – a particle with no charge, but a mass nearly equal to a proton He was trying to identify the extra mass of an atomic nucleus by firing alpha particles into a beryllium target and allowing the resulting radiation to interact with paraffin wax. The ...
Endothermic And Exothermic Reactions
... that absorbs energy from its surroundings. More energy is required to break the bonds in the reactants than is released by the formation of bonds in the products. In these reactions, heat is shown as one This is a typical graph of an of the reactants endothermic reaction with the A + B + heat C pr ...
... that absorbs energy from its surroundings. More energy is required to break the bonds in the reactants than is released by the formation of bonds in the products. In these reactions, heat is shown as one This is a typical graph of an of the reactants endothermic reaction with the A + B + heat C pr ...
end of year review
... A cube of an unknown metal has a volume of 2.25 cm3 and a mass of 16.2 g. Based on data in the table above, what is the identity of this metal? a. bismuth ...
... A cube of an unknown metal has a volume of 2.25 cm3 and a mass of 16.2 g. Based on data in the table above, what is the identity of this metal? a. bismuth ...
CHEMICAL REACTIONS OBJECTIVES 1. To study reactions
... reaction will be observed. For example, no reaction occurs between NaCl and KNO3 because all ionic combinations are soluble compounds. Part II. Exothermic and endothermic reactions Reactions that evolve heat are called exothermic reactions. In an exothermic reaction, the products have less energy th ...
... reaction will be observed. For example, no reaction occurs between NaCl and KNO3 because all ionic combinations are soluble compounds. Part II. Exothermic and endothermic reactions Reactions that evolve heat are called exothermic reactions. In an exothermic reaction, the products have less energy th ...