15.0 EquilibriumIHS2014
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
Stoichiometry Help II
... Stoichiometry is a step by step process. You will be shown each step. At the end of each section you will need to do the practice problems to see that you are on the right track. At any time you may go back if you are lost. It is very important that you understand a step before moving on to the ...
... Stoichiometry is a step by step process. You will be shown each step. At the end of each section you will need to do the practice problems to see that you are on the right track. At any time you may go back if you are lost. It is very important that you understand a step before moving on to the ...
Topic 1 - Coral Gables Senior High
... theory. This proposed the existence of a fire-like element that was released during these processes. The theory seemed to explain some of the observations of its time, although these were purely qualitative. It could not explain later quantitative data showing that substances actually gain rather th ...
... theory. This proposed the existence of a fire-like element that was released during these processes. The theory seemed to explain some of the observations of its time, although these were purely qualitative. It could not explain later quantitative data showing that substances actually gain rather th ...
Chapter
... • Polyatomic ions are single ions that contain more than one atom • Often identified by (ion) in formula • Name and charge of polyatomic ion do not change • Name any ionic compound by naming cation first and then anion Tro, Chemistry: A Molecular Approach ...
... • Polyatomic ions are single ions that contain more than one atom • Often identified by (ion) in formula • Name and charge of polyatomic ion do not change • Name any ionic compound by naming cation first and then anion Tro, Chemistry: A Molecular Approach ...
Chapter
... • Polyatomic ions are single ions that contain more than one atom • Often identified by (ion) in formula • Name and charge of polyatomic ion do not change • Name any ionic compound by naming cation first and then anion Tro, Chemistry: A Molecular Approach ...
... • Polyatomic ions are single ions that contain more than one atom • Often identified by (ion) in formula • Name and charge of polyatomic ion do not change • Name any ionic compound by naming cation first and then anion Tro, Chemistry: A Molecular Approach ...
Study Guide for Content Mastery - Student Edition
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
Stoichiometry notes 1
... 1. Write a balanced chemical equation. 2. Label your given and target substances. 3. Convert your given unit(s) to moles of given substance using the appropriate conversion factor. 4. Convert moles of given substance to moles of target substance using the mole ratio from the balanced equation. 5. Co ...
... 1. Write a balanced chemical equation. 2. Label your given and target substances. 3. Convert your given unit(s) to moles of given substance using the appropriate conversion factor. 4. Convert moles of given substance to moles of target substance using the mole ratio from the balanced equation. 5. Co ...
Chapter 3 - Educator
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
Ch. 12 Stoichiometry
... Pay attention to the substance and units asked for in the problem......we need grams of CO2. Step 2: Find the ratio between the unknown substance and the known substance in the problem: ...
... Pay attention to the substance and units asked for in the problem......we need grams of CO2. Step 2: Find the ratio between the unknown substance and the known substance in the problem: ...
Document
... # Vacancy defect – When some of the lattice sides are vacant, the crystal is said to have vacancy defect. This results the decrease in density of the substance. This defect develops when a substance is heated. # Interstitial defect- When some constituent particles occupy an interstitial site, the cr ...
... # Vacancy defect – When some of the lattice sides are vacant, the crystal is said to have vacancy defect. This results the decrease in density of the substance. This defect develops when a substance is heated. # Interstitial defect- When some constituent particles occupy an interstitial site, the cr ...
Stoichiometry of Ozonation of Environmentally
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
CHOICE BASED CREDIT SYSTEM B. Sc. WITH CHEMISTRY
... and angular parts of the hydogenic wavefunctions (atomic orbitals) and their variations for 1s, 2s, 2p, 3s, 3p and 3d orbitals (Only graphical representation). Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special re ...
... and angular parts of the hydogenic wavefunctions (atomic orbitals) and their variations for 1s, 2s, 2p, 3s, 3p and 3d orbitals (Only graphical representation). Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special re ...
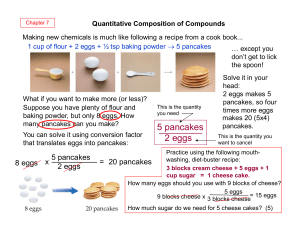
5 pancakes 2 eggs
... What if you want to make more (or less)? Suppose you have plenty of flour and baking powder, but only 8 eggs. How many pancakes can you make? ...
... What if you want to make more (or less)? Suppose you have plenty of flour and baking powder, but only 8 eggs. How many pancakes can you make? ...
Chapter 3: Calculations with Chemical Formulas
... consumed but before all the Al is consumed. The limiting reactant is therefore HCl. The amount of AlCl3 produced must be 0.1166, or 0.12 mol. ...
... consumed but before all the Al is consumed. The limiting reactant is therefore HCl. The amount of AlCl3 produced must be 0.1166, or 0.12 mol. ...
chem textbook 2015 - Manitowoc Public School District
... This generally means that your notes are incomplete, meaning that you wrote down much of what was on the board but did not record any of the verbal discussion or rationale used to explain what was taking place. It is important that your notes include your thoughts rather than just what I right on th ...
... This generally means that your notes are incomplete, meaning that you wrote down much of what was on the board but did not record any of the verbal discussion or rationale used to explain what was taking place. It is important that your notes include your thoughts rather than just what I right on th ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
... conductivity of the products can be used to measure reaction rate. This method is usually used when non-ionic reactants form ionic products (Silberberg 681). Reaction rate can be calculated by finding the change in formation of product over time, or by finding the change in consumption of a reactant ...
Periodic table, elements and physical chemistry
... identify and contact all copyright holders whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements booklet. This is produced for each series of examinations and ...
... identify and contact all copyright holders whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements booklet. This is produced for each series of examinations and ...
Kinetics
... formation of an activated complex, where bonds are breaking and new ones forming. When temperature is increased, a greater number of molecular collisions possess enough energy to activate the reaction (activation energy). Frequency of collisions increases - an increase in temperature makes particles ...
... formation of an activated complex, where bonds are breaking and new ones forming. When temperature is increased, a greater number of molecular collisions possess enough energy to activate the reaction (activation energy). Frequency of collisions increases - an increase in temperature makes particles ...
Stoichiometry
... + 7 O2 -----> 4 CO2 + 6 H2O 6. During its combustion, ethane C2H6, combines with oxygen O2 to give carbon dioxide and water. A sample of ethane was burned completely and the water that formed has a mass of 1.61 grams. How many grams of ethane was in the sample? 0.90 grams of ethane ...
... + 7 O2 -----> 4 CO2 + 6 H2O 6. During its combustion, ethane C2H6, combines with oxygen O2 to give carbon dioxide and water. A sample of ethane was burned completely and the water that formed has a mass of 1.61 grams. How many grams of ethane was in the sample? 0.90 grams of ethane ...
Thermochemistry Diploma Questions
... Ethanol is the alcohol found in beer, wine, and whiskey. In the production of ethanol, the starch in barley, grapes, or corn is reacted to form glucose in the presence of enzymes. During the fermentation process, yeast is added to the glucose. The yeast contains enzymes that act as biological cataly ...
... Ethanol is the alcohol found in beer, wine, and whiskey. In the production of ethanol, the starch in barley, grapes, or corn is reacted to form glucose in the presence of enzymes. During the fermentation process, yeast is added to the glucose. The yeast contains enzymes that act as biological cataly ...