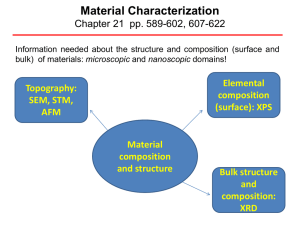
Chapter 7
... temperature will increase the reaction rate. • Ex: You store milk in a refrigerator to slow down the reactions that cause the milk to spoil • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and mo ...
... temperature will increase the reaction rate. • Ex: You store milk in a refrigerator to slow down the reactions that cause the milk to spoil • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and mo ...
Determining Density through graphing
... In the first week, she studied daily for 15 minutes and her end of the week test scores were 60%. During the second week, she studied daily for 30 minutes and her end of the week test scores were 70%. During the third week, she studied for 45 minutes and her end of the week test scores were 80%. Fin ...
... In the first week, she studied daily for 15 minutes and her end of the week test scores were 60%. During the second week, she studied daily for 30 minutes and her end of the week test scores were 70%. During the third week, she studied for 45 minutes and her end of the week test scores were 80%. Fin ...
An Overview of Organic Reactions
... Each new center may generate a potential pair of stereoisomers, so the theoretical number of possible stereoisomers is 2 n (May have fewer if symmetry elements are present) Relationship of 1 and 2 = enantiomers l Enantiomers = same properties, cannot be separated ...
... Each new center may generate a potential pair of stereoisomers, so the theoretical number of possible stereoisomers is 2 n (May have fewer if symmetry elements are present) Relationship of 1 and 2 = enantiomers l Enantiomers = same properties, cannot be separated ...
Lesson Summary
... But how did scientists gather evidence about something too small to be seen? Scientists found they could learn more about atoms and their structure by shooting small pieces of matter at them or by heating them in a flame. Observations from these experiments provided evidence that helped scientists m ...
... But how did scientists gather evidence about something too small to be seen? Scientists found they could learn more about atoms and their structure by shooting small pieces of matter at them or by heating them in a flame. Observations from these experiments provided evidence that helped scientists m ...
4.2 reading
... atoms have 8 neutrons and a mass number of 16. Some oxygen atoms twice the mass of hydrogen-1 have 9 neutrons and a mass number of 17. Some oxygen atoms have atoms. Using Tables At what 10 neutrons and a mass number of 18. When it is important to distintemperature would a sample of heavy water freez ...
... atoms have 8 neutrons and a mass number of 16. Some oxygen atoms twice the mass of hydrogen-1 have 9 neutrons and a mass number of 17. Some oxygen atoms have atoms. Using Tables At what 10 neutrons and a mass number of 18. When it is important to distintemperature would a sample of heavy water freez ...
2016
... a. Corrosion of aluminum metal. b. Melting of ice. c. Pulverizing an aspirin. d. Digesting a candy bar. e. Explosion of nitroglycerin. 13. You may notice when water boils, you can see bubbles that rise to the surface of the water. a. What is inside these bubbles? b. Is the boiling of water a chemica ...
... a. Corrosion of aluminum metal. b. Melting of ice. c. Pulverizing an aspirin. d. Digesting a candy bar. e. Explosion of nitroglycerin. 13. You may notice when water boils, you can see bubbles that rise to the surface of the water. a. What is inside these bubbles? b. Is the boiling of water a chemica ...
matter - Firelands Local Schools
... The number after and below the atomic symbol indicates the number of that element 1. Example: C16H10N2O2 = 16 carbon, 10 hydrogen, 2 nitrogen, and 2 oxygen ...
... The number after and below the atomic symbol indicates the number of that element 1. Example: C16H10N2O2 = 16 carbon, 10 hydrogen, 2 nitrogen, and 2 oxygen ...
atomic mass - Belle Vernon Area School District
... electron, but opposite in sign, called protons Protons are found in the nucleus and have a charge=+1.60 x1019 C and mass=1.67262 x 10−24 g Each proton has a charge of +1 charge units (cu) protons and electrons have equal amounts of charge, but opposite in sign, therefore atoms must have equal ...
... electron, but opposite in sign, called protons Protons are found in the nucleus and have a charge=+1.60 x1019 C and mass=1.67262 x 10−24 g Each proton has a charge of +1 charge units (cu) protons and electrons have equal amounts of charge, but opposite in sign, therefore atoms must have equal ...
Balanced Chemical Equation
... • Add OH− ions to both sides of the equation in numbers equal to the number of H+ ions. • On the side of the equation containing both H+ and OH− ions, combine these ions to yield water molecules. • Simplify the equation by removing any redundant water molecules. 9. Finally, check to see that both th ...
... • Add OH− ions to both sides of the equation in numbers equal to the number of H+ ions. • On the side of the equation containing both H+ and OH− ions, combine these ions to yield water molecules. • Simplify the equation by removing any redundant water molecules. 9. Finally, check to see that both th ...
The History of the Atom - cho
... Chemistry CPS: Project #2: GUIDELINES The History of the Atom: NAMES OF THE SCIENTISTS:__________________________________________________ _____________________________________________________________ Date assigned: ______________________________ Date due: __________________________________ P ...
... Chemistry CPS: Project #2: GUIDELINES The History of the Atom: NAMES OF THE SCIENTISTS:__________________________________________________ _____________________________________________________________ Date assigned: ______________________________ Date due: __________________________________ P ...
C:\my documents\6th Grade Science\Matter\Models
... Because Dalton’s model did not agree with experimental evidence, it was necessary to change the model of an atom. Thomson revised Dalton’s model from a uniform solid ball to a sphere of positive charge with electrons spread evenly among the positive charge. Below is an example of Thomson’s model – t ...
... Because Dalton’s model did not agree with experimental evidence, it was necessary to change the model of an atom. Thomson revised Dalton’s model from a uniform solid ball to a sphere of positive charge with electrons spread evenly among the positive charge. Below is an example of Thomson’s model – t ...
Models of the Atom A Brief History First Thoughts For over 2500
... Because Dalton’s model did not agree with experimental evidence, it was necessary to change the model of an atom. Thomson revised Dalton’s model from a uniform solid ball to a sphere of positive charge with electrons spread evenly among the positive charge. Below is an example of Thomson’s model – t ...
... Because Dalton’s model did not agree with experimental evidence, it was necessary to change the model of an atom. Thomson revised Dalton’s model from a uniform solid ball to a sphere of positive charge with electrons spread evenly among the positive charge. Below is an example of Thomson’s model – t ...
Chapter 6
... benzoic acid (HC7H5O2), a weak acid that has one acidic hydrogen atom per molecule. A sample of the effluent weighing 0.3518 g was shaken with water, and the resulting aqueous solution required 10.59 mL of 0.1546 M NaOH for neutralization. Calculate the mass percent of HC7H5O2 in the original sample ...
... benzoic acid (HC7H5O2), a weak acid that has one acidic hydrogen atom per molecule. A sample of the effluent weighing 0.3518 g was shaken with water, and the resulting aqueous solution required 10.59 mL of 0.1546 M NaOH for neutralization. Calculate the mass percent of HC7H5O2 in the original sample ...
NYOS Charter School
... 10. For a given reaction conducted at -150 C, the following data was collected: H = +2.5 kJ and S = +315 J/K. Calculate G using the Gibb’s Free Energy equation and express your answer in joules. You must show your work for full credit. ...
... 10. For a given reaction conducted at -150 C, the following data was collected: H = +2.5 kJ and S = +315 J/K. Calculate G using the Gibb’s Free Energy equation and express your answer in joules. You must show your work for full credit. ...
Ch 11 HW
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
1 Structure of Atom - Viva Online Learning
... We know that under ordinary conditions, gases are poor conductor of electricity. But gases become good conductor of electricity when (i) the gas is stored at very low pressure (0.01–0.001 mm of mercury) and (ii) a very high voltage is applied through the gas (more than 10,000 volts). J.J. Thomson ap ...
... We know that under ordinary conditions, gases are poor conductor of electricity. But gases become good conductor of electricity when (i) the gas is stored at very low pressure (0.01–0.001 mm of mercury) and (ii) a very high voltage is applied through the gas (more than 10,000 volts). J.J. Thomson ap ...
Structure of the atom,english2009-08
... Indicate form and positition of orbital as probability of electron arrangement and show the sub level of energy. Azimut quantum number follow equal l = n – 1 Azimut quantum number have value from 0 to n – 1 ...
... Indicate form and positition of orbital as probability of electron arrangement and show the sub level of energy. Azimut quantum number follow equal l = n – 1 Azimut quantum number have value from 0 to n – 1 ...
Atomic Structure - Miami East Local Schools
... simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or rearranging atoms. ...
... simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or rearranging atoms. ...
Review 1
... The calculated density of the figurine is less than the value for silver. This does not conclusively prove the figurine is made of another metal. The figurine could be pure silver but hollow. It also might be an alloy of silver and another, less dense metal. ...
... The calculated density of the figurine is less than the value for silver. This does not conclusively prove the figurine is made of another metal. The figurine could be pure silver but hollow. It also might be an alloy of silver and another, less dense metal. ...
chapter 1 - Revsworld
... Which of the following statements is/are correct? I. When heat energy flows from a system to the surroundings, we know that the temperature of the system is greater than that of the surroundings. II. Given the thermochemical equation 4NH3(g) + 5O2(g) ------> 4 NO(g) + 6H2O(g) H = -906 kJ, the therm ...
... Which of the following statements is/are correct? I. When heat energy flows from a system to the surroundings, we know that the temperature of the system is greater than that of the surroundings. II. Given the thermochemical equation 4NH3(g) + 5O2(g) ------> 4 NO(g) + 6H2O(g) H = -906 kJ, the therm ...
Describe properties of particles and thermochemical - Mr
... NOTE: Atoms of elements with widely different electronegativities tend to form ionic bonds with each other since the atom of the less electronegative element gives up its electron(s) to the atom of the more electronegative element. Atoms of elements with more similar electronegativities tend to form ...
... NOTE: Atoms of elements with widely different electronegativities tend to form ionic bonds with each other since the atom of the less electronegative element gives up its electron(s) to the atom of the more electronegative element. Atoms of elements with more similar electronegativities tend to form ...
File
... Example Exercise 4.1 Atomic Notation Continued b. Because the atomic number is 47 and the mass number is 109, the number of neutrons is 62 (109 − 47). If there are 47 protons, there must be 47 electrons. ...
... Example Exercise 4.1 Atomic Notation Continued b. Because the atomic number is 47 and the mass number is 109, the number of neutrons is 62 (109 − 47). If there are 47 protons, there must be 47 electrons. ...