Chapter 4 Quantities of Reactants and Products 4.1 Chemical
... ____________________ are the numbers placed in front of formulas in a chemical equation to balance the equation, thereby indicating the combining ratios of the reactants and the products. Balanced equations obey the law of conservation of mass. That is the total mass before a reaction takes place wi ...
... ____________________ are the numbers placed in front of formulas in a chemical equation to balance the equation, thereby indicating the combining ratios of the reactants and the products. Balanced equations obey the law of conservation of mass. That is the total mass before a reaction takes place wi ...
Formula and The Mole
... 200cm3 of solution, concentration 0.5mol l-1? 7. What is the concentration of a solution of a solution which contains 2 mol of hydrogen chloride dissolved and made up to 2 litres of solution? 8. What volume of a solution, concentration 0.2 mol l-1, contains 0.005 mol of solute? 9. 52.5g of pure citr ...
... 200cm3 of solution, concentration 0.5mol l-1? 7. What is the concentration of a solution of a solution which contains 2 mol of hydrogen chloride dissolved and made up to 2 litres of solution? 8. What volume of a solution, concentration 0.2 mol l-1, contains 0.005 mol of solute? 9. 52.5g of pure citr ...
CHEMISTRY 110 LECTURE
... 6. Iron (III) oxide can react with aluminum metal to produce aluminum oxide and iron metal (hint: this is the chemical rxn!!) This is called the thermit reaction and it produces so much heat that it can be used for incendiary bombs and for welding. How many grams of aluminum oxide will be produced b ...
... 6. Iron (III) oxide can react with aluminum metal to produce aluminum oxide and iron metal (hint: this is the chemical rxn!!) This is called the thermit reaction and it produces so much heat that it can be used for incendiary bombs and for welding. How many grams of aluminum oxide will be produced b ...
N5 Chemistry Course Specification 2017-18 session
... The mass number of an atom is equal to the number of protons added to the number of neutrons. Isotopes are defined as atoms with the same atomic number but different mass numbers, or as atoms with the same number of protons but different numbers of neutrons. Nuclide notation is used to show the atom ...
... The mass number of an atom is equal to the number of protons added to the number of neutrons. Isotopes are defined as atoms with the same atomic number but different mass numbers, or as atoms with the same number of protons but different numbers of neutrons. Nuclide notation is used to show the atom ...
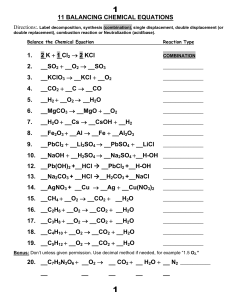
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... Directions - Write the following reactions equation noting the states. For example, note a gas as (g). Then balance the equation by placing coefficients in front of the formula. For example, 2 CO2. ...
... Directions - Write the following reactions equation noting the states. For example, note a gas as (g). Then balance the equation by placing coefficients in front of the formula. For example, 2 CO2. ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Answers to Selected Questions and Problems
... (e) energy; (f) physical property; (g) liquid; (h) density; (i) homogeneous mixture; (j) solid (a) 2.95 × 104; (b) 8.2 × 10−5; (c) 6.5 × 108; (d) 1.00 × 10−2 (a) 0.0000186; (b) 10,000,000; (c) 453,000; (d) 0.0061 (a) 6.2 × 103; (b) 3.5 × 107; (c) 2.9 × 10−3; (d) 2.5 × 10−7; (e) 8.20 × 105; (f) 1.6 × ...
... (e) energy; (f) physical property; (g) liquid; (h) density; (i) homogeneous mixture; (j) solid (a) 2.95 × 104; (b) 8.2 × 10−5; (c) 6.5 × 108; (d) 1.00 × 10−2 (a) 0.0000186; (b) 10,000,000; (c) 453,000; (d) 0.0061 (a) 6.2 × 103; (b) 3.5 × 107; (c) 2.9 × 10−3; (d) 2.5 × 10−7; (e) 8.20 × 105; (f) 1.6 × ...
Document
... temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again? • To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves t ...
... temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again? • To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves t ...
One-electron atom Notes on Quantum Mechanics
... There are two features of the one-electron atom that allow us to simplify our analysis. First, because the nucleus is so much heavier than the electron, to e very good approximation we can treat the nucleus as fixed in space, with the electron moving around it. Second, because there are no other ele ...
... There are two features of the one-electron atom that allow us to simplify our analysis. First, because the nucleus is so much heavier than the electron, to e very good approximation we can treat the nucleus as fixed in space, with the electron moving around it. Second, because there are no other ele ...
Stoichometry Notes (Unit 2)
... The total number of atoms of each element (and the sum of their respective masses) on the reactant side of the “à” must be equal to the total number of atoms of each element (and the sum of their respective masses) on the product side. Chemical equations frequently contain additional symbols to repr ...
... The total number of atoms of each element (and the sum of their respective masses) on the reactant side of the “à” must be equal to the total number of atoms of each element (and the sum of their respective masses) on the product side. Chemical equations frequently contain additional symbols to repr ...
atom
... Periodic Chart You can also find these symbols and names in the periodic table inside the front cover of your text although this chart does not have names provided. ...
... Periodic Chart You can also find these symbols and names in the periodic table inside the front cover of your text although this chart does not have names provided. ...
TEKS Presentation Properties of Matter
... How the specific heat of water affects the Earth Oceans cover about 2/3 of Earth’s surface. Water’s characteristic of retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
... How the specific heat of water affects the Earth Oceans cover about 2/3 of Earth’s surface. Water’s characteristic of retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
An Artist`s Modest Proposal
... Niels Bohr's atom model of 1913 was abandoned by science over eighty years ago yet it is still introduced in all science classrooms and it remains famous the world over as the cartoon-symbol meaning "atom". In the dramatic causal/acausal debates of the 1920s, the Copenhagen people who argued to disa ...
... Niels Bohr's atom model of 1913 was abandoned by science over eighty years ago yet it is still introduced in all science classrooms and it remains famous the world over as the cartoon-symbol meaning "atom". In the dramatic causal/acausal debates of the 1920s, the Copenhagen people who argued to disa ...
General Chemistry Questions
... 6. Two solutions (the system), each of 25.0 mL volume and at 25.0 °C, are mixed in a beaker. A reaction occurs between them, causing the temperature to drop to 20.0 °C. After the products have equilibrated with the surroundings, the temperature is again 25.0 °C and the total volume is 50.0 mL. No ga ...
... 6. Two solutions (the system), each of 25.0 mL volume and at 25.0 °C, are mixed in a beaker. A reaction occurs between them, causing the temperature to drop to 20.0 °C. After the products have equilibrated with the surroundings, the temperature is again 25.0 °C and the total volume is 50.0 mL. No ga ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
2 Atomic structure
... central nucleus (Figure 2.1). The nucleus is made up of protons and neutrons (except for a hydrogen atom, which has no neutrons). The actual mass of a proton is 1.67 × 10−27 kg and the charge on a proton is +1.6 × 10−19 C. Relative masses and charges, shown in Table 2.1, are used to compare the mass ...
... central nucleus (Figure 2.1). The nucleus is made up of protons and neutrons (except for a hydrogen atom, which has no neutrons). The actual mass of a proton is 1.67 × 10−27 kg and the charge on a proton is +1.6 × 10−19 C. Relative masses and charges, shown in Table 2.1, are used to compare the mass ...
Chemical Reactions
... the sodium hydroxide that is important and that the moles of H2SO4 did not change, only the concentration.) 5. Fill a buret with barium hydroxide. 6. Place the beaker under the buret and drop the buret tip so that it is just below the mouth of the beaker. 7. Place the electrodes down into the beaker ...
... the sodium hydroxide that is important and that the moles of H2SO4 did not change, only the concentration.) 5. Fill a buret with barium hydroxide. 6. Place the beaker under the buret and drop the buret tip so that it is just below the mouth of the beaker. 7. Place the electrodes down into the beaker ...
Honors Chemistry Worksheet – Atomic Theory and the Basic Atom
... Neither as gamma rays possess no charge and neutrons possess no charge meaning neither would interact with the applied magnetic field. 20. Two beams of ions; germanium – 74 and selenium – 74 pass between a pair of charged plates. The ions have the same charge, but the velocity of the germanium ions ...
... Neither as gamma rays possess no charge and neutrons possess no charge meaning neither would interact with the applied magnetic field. 20. Two beams of ions; germanium – 74 and selenium – 74 pass between a pair of charged plates. The ions have the same charge, but the velocity of the germanium ions ...
Chemical Bonding
... nuclei. The repulsions between the nuclei increase because they are poorly shielded from each other, increasing the energy and decreasing the stability of the molecule. ...
... nuclei. The repulsions between the nuclei increase because they are poorly shielded from each other, increasing the energy and decreasing the stability of the molecule. ...
chemistry
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...