Chapter 5: What you should know when you finish. Describe the
... Magnesium and calcium have essential biological functions and they provide materials used in construction and transportation. Magnesium plays a key role in the process that uses sunlight to produce sugar in plants A mixture of magnesium and other metals can be as strong as steel, but much ligh ...
... Magnesium and calcium have essential biological functions and they provide materials used in construction and transportation. Magnesium plays a key role in the process that uses sunlight to produce sugar in plants A mixture of magnesium and other metals can be as strong as steel, but much ligh ...
Periodic Table WebQuest
... 9. Is the quantity of protons always equal to the quantity of neutrons in an atom? Explain. 10. List 3 uses of isotopes. 11. Describe how scientists calculate the average atomic mass of an element. Use http://www.chem4kids.com/files/elem_intro.html, http://www.chem4kids.com/files/elem_transmetal.ht ...
... 9. Is the quantity of protons always equal to the quantity of neutrons in an atom? Explain. 10. List 3 uses of isotopes. 11. Describe how scientists calculate the average atomic mass of an element. Use http://www.chem4kids.com/files/elem_intro.html, http://www.chem4kids.com/files/elem_transmetal.ht ...
Chapter Test B
... ease with which an atom gains electrons. Electronegativity is a measure of the ability of an atom to attract electrons. Therefore, atoms with a high negative electron affinity are also the most electronegative. 26. The physical and chemical properties of the elements are periodic functions of their ...
... ease with which an atom gains electrons. Electronegativity is a measure of the ability of an atom to attract electrons. Therefore, atoms with a high negative electron affinity are also the most electronegative. 26. The physical and chemical properties of the elements are periodic functions of their ...
Ch.4 Notes Powerpoint Version
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
... Adjusting the Periodic Table • About 40 years after Mendeleev’s table, Henry Moseley made an important change to the periodic table: ▫ Organized elements by atomic number instead of atomic mass. ▫ Elements that had not previously fit into the correct column when ordered by atomic mass were fixed. ...
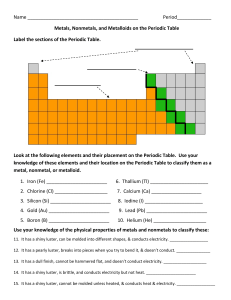
Name Period_____________ Metals, Nonmetals, and Metalloids on
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
Periodic table of elements
... The compounds of transition metals are usually brightly colored and are often used to color paints. Transition elements have 1 or 2 valence electrons, which they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to-outermost level. ...
... The compounds of transition metals are usually brightly colored and are often used to color paints. Transition elements have 1 or 2 valence electrons, which they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to-outermost level. ...
the periodic table
... CHEMISTRY TEST REVIEW Use this as a study tool to review for your CCA, October 18 th. ...
... CHEMISTRY TEST REVIEW Use this as a study tool to review for your CCA, October 18 th. ...
Study Guide for Electrons Mini-Test - seys
... Period: a horizontal row in the periodic table = properties of elements change in a predictable way from one end of a period to the other Family: used to describe elements that share certain characteristics—not only in terms of observable behavior, but also with regard to atomic structure (electron ...
... Period: a horizontal row in the periodic table = properties of elements change in a predictable way from one end of a period to the other Family: used to describe elements that share certain characteristics—not only in terms of observable behavior, but also with regard to atomic structure (electron ...
Enriched Chemistry Chapter 5 * The Periodic Law
... Mendeleev’s periodic table grouped elements by their properties. • Mendeleev placed the name of each known element on a card, with the atomic mass and the observed phys/chem properties • He then arranged them in various configurations, looking for patterns or trends • He noticed that when the eleme ...
... Mendeleev’s periodic table grouped elements by their properties. • Mendeleev placed the name of each known element on a card, with the atomic mass and the observed phys/chem properties • He then arranged them in various configurations, looking for patterns or trends • He noticed that when the eleme ...
Periodic Properties of the Elements
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
Name - Haverford Alchemy
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
The Periodic Table of Elements
... 8. Which of the following is a property of aqueous potassium iodide? a. it does not conduct electricity b. it is decolorised by chlorine c. it reacts with aqueous bromine to form iodine d. it reacts with aqueous lead(II) nitrate to form a white precipitate 9. Many properties of an element can its co ...
... 8. Which of the following is a property of aqueous potassium iodide? a. it does not conduct electricity b. it is decolorised by chlorine c. it reacts with aqueous bromine to form iodine d. it reacts with aqueous lead(II) nitrate to form a white precipitate 9. Many properties of an element can its co ...
The periodic table
... • Another problem was that the noble gases had not been discovered and when we count these we know the repetition in properties actually occurs every 9th element • Newlands was treated very badly by his fellow scientists but 20 years later was awarded a medal when it was found his ideas were basica ...
... • Another problem was that the noble gases had not been discovered and when we count these we know the repetition in properties actually occurs every 9th element • Newlands was treated very badly by his fellow scientists but 20 years later was awarded a medal when it was found his ideas were basica ...
Unit Bohr Model and Electromagnetic Radiation - Jones
... Chm.1.3 Understand the physical and chemical properties of atoms based on their position on the Periodic Table. Chm.1.3.1 Classify the components of a periodic table (period, group, metal, metalloid, nonmetal, transition). Chm.1.3.1 Using the Periodic Table, ...
... Chm.1.3 Understand the physical and chemical properties of atoms based on their position on the Periodic Table. Chm.1.3.1 Classify the components of a periodic table (period, group, metal, metalloid, nonmetal, transition). Chm.1.3.1 Using the Periodic Table, ...
Periodic classificatiion of elements
... A. Limitations of Newlands classification are a. Newland’s law was applicable only upto calcium. 1. He assumed that only 56 elements existed in nature and no more elements would be discovered in future. 2. Cobalt [Co] and Nickel [Ni] are placed in the same slot and these are placed in the same colu ...
... A. Limitations of Newlands classification are a. Newland’s law was applicable only upto calcium. 1. He assumed that only 56 elements existed in nature and no more elements would be discovered in future. 2. Cobalt [Co] and Nickel [Ni] are placed in the same slot and these are placed in the same colu ...
Atoms, Elements, and the Periodic Table Spring Packet
... period (or row) in the periodic table? (Example: Na Ar) ...
... period (or row) in the periodic table? (Example: Na Ar) ...
7.1 The Periodic Table
... • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physical properties. ...
... • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physical properties. ...
Periodic Relationships Among the Elements
... Discovery of the 6 Noble Gases (1898 – 1900) • Difficult to detect due to inert nature • Ramsay reacted N2(g) with Mg(s) to form Mg3N2(s) along with a volume of unknown gas that would not react. • Using a discharge tube, they noted the atomic line emission spectrum was unique. • It was called Argon ...
... Discovery of the 6 Noble Gases (1898 – 1900) • Difficult to detect due to inert nature • Ramsay reacted N2(g) with Mg(s) to form Mg3N2(s) along with a volume of unknown gas that would not react. • Using a discharge tube, they noted the atomic line emission spectrum was unique. • It was called Argon ...
AP Chemistry Chapter 7
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their at ...
... *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their at ...
Chemistry 4.1 Atomic structure and the periodic table NEED TO
... The elements in the periodic table are arranged in order of atomic (proton) number and so that elements with similar properties are in columns, known as groups. The table is called a periodic table because similar properties occur at regular intervals. Elements in the same group in the periodic tabl ...
... The elements in the periodic table are arranged in order of atomic (proton) number and so that elements with similar properties are in columns, known as groups. The table is called a periodic table because similar properties occur at regular intervals. Elements in the same group in the periodic tabl ...
File
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.