The Periodic Table
... Elements in Group 6 only need two more electrons to fill their outer level. Elements in Group 7 only need one more electron to fill their outer level. ...
... Elements in Group 6 only need two more electrons to fill their outer level. Elements in Group 7 only need one more electron to fill their outer level. ...
Unit 3-The Big Picture
... chemical properties show a periodic pattern. Vertical columns called groups have similar properties because of their similar valence electron configurations. Horizontal rows called periods have predictable properties based on an increasing number of electrons in the outer orbitals. The names of grou ...
... chemical properties show a periodic pattern. Vertical columns called groups have similar properties because of their similar valence electron configurations. Horizontal rows called periods have predictable properties based on an increasing number of electrons in the outer orbitals. The names of grou ...
Periodic Properties of the Elements
... Additional chemical and physical trends among the table’s constituents can be understood by ‘popping the hood’ on these elements and determining the relationship between atomic properties electronic structures ...
... Additional chemical and physical trends among the table’s constituents can be understood by ‘popping the hood’ on these elements and determining the relationship between atomic properties electronic structures ...
Unit 1: Introduction to Chemistry Directions: Use your notes
... atomic number, repetitious trends can be seen. Mendeleev’s periodic table was arranged in order of increasing atomic mass. He then arranged columns in order to have elements with similar properties align in columns. The modern table is arranged by atomic number. a. What subatomic particle decides th ...
... atomic number, repetitious trends can be seen. Mendeleev’s periodic table was arranged in order of increasing atomic mass. He then arranged columns in order to have elements with similar properties align in columns. The modern table is arranged by atomic number. a. What subatomic particle decides th ...
periodic table
... water to from an alkaline solution – Group IIA are called the alkaline earth metals because they are reactive, but not as reactive as Group IA. • They are also soft metals like Earth. ...
... water to from an alkaline solution – Group IIA are called the alkaline earth metals because they are reactive, but not as reactive as Group IA. • They are also soft metals like Earth. ...
Unit 7 Periodic Properties of the Elements in the Periodic Table
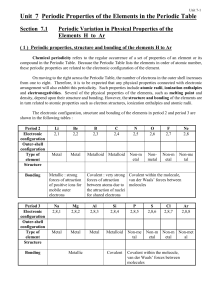
... Across the period, the first ionization enthalpy generally increases because the effective nuclear charge increases giving rise to a decrease in atomic radius and thus a decrease in the distance of the electron cloud. As the electron cloud becomes closer to the nucleus, it is more difficult to remov ...
... Across the period, the first ionization enthalpy generally increases because the effective nuclear charge increases giving rise to a decrease in atomic radius and thus a decrease in the distance of the electron cloud. As the electron cloud becomes closer to the nucleus, it is more difficult to remov ...
Chemistry Week 16
... Aufbau rule, Hund’s rule, and the Pauli exclusion principle that determine a probable location of an atom’s electrons. Students will go over the answers to the unit 5 test and rework the problems that they missed. ...
... Aufbau rule, Hund’s rule, and the Pauli exclusion principle that determine a probable location of an atom’s electrons. Students will go over the answers to the unit 5 test and rework the problems that they missed. ...
Unit 1 - PDF Format
... radioactive are known as radioisotopes An element may have one or more isotopes which are not radioactive in addition to a radioisotope ...
... radioactive are known as radioisotopes An element may have one or more isotopes which are not radioactive in addition to a radioisotope ...
Unit 1
... radioactive are known as radioisotopes An element may have one or more isotopes which are not radioactive in addition to a radioisotope ...
... radioactive are known as radioisotopes An element may have one or more isotopes which are not radioactive in addition to a radioisotope ...
Unit 3.2: The Periodic Table and Periodic Trends Notes
... Groups 3 through 12 contain transition elements. The transition metals are harder & less reactive than Group 1 & 2 metals. Because the outer shells of these elements are filling the d-orbital, they are sometimes called d-block elements. ...
... Groups 3 through 12 contain transition elements. The transition metals are harder & less reactive than Group 1 & 2 metals. Because the outer shells of these elements are filling the d-orbital, they are sometimes called d-block elements. ...
Metals and Nonmetals Metals and Nonmetals
... increase while the n stays the same. • For transition metals the electrons are going into an INNER shell so the screening is more pronounced. The number of outer shell electrons stays the same. The increase in the nuclear charge is balanced by the increased screening. The outer electrons see the sam ...
... increase while the n stays the same. • For transition metals the electrons are going into an INNER shell so the screening is more pronounced. The number of outer shell electrons stays the same. The increase in the nuclear charge is balanced by the increased screening. The outer electrons see the sam ...
Metals and Nonmetals
... increase while the n stays the same. • For transition metals the electrons are going into an INNER shell so the screening is more pronounced. The number of outer shell electrons stays the same. The increase in the nuclear charge is balanced by the increased screening. The outer electrons see the sam ...
... increase while the n stays the same. • For transition metals the electrons are going into an INNER shell so the screening is more pronounced. The number of outer shell electrons stays the same. The increase in the nuclear charge is balanced by the increased screening. The outer electrons see the sam ...
Trends in the Periodic Table_Notes
... Linus Pauling, an American chemist (and winner of two Nobel prizes!) came up with the concept of electronegativity in 1932 to help explain the nature of chemical bonds. Since fluorine is the most electronegative element (has the greatest attraction for the bonding electrons) he assigned it a value a ...
... Linus Pauling, an American chemist (and winner of two Nobel prizes!) came up with the concept of electronegativity in 1932 to help explain the nature of chemical bonds. Since fluorine is the most electronegative element (has the greatest attraction for the bonding electrons) he assigned it a value a ...
How can atomic theory explain patterns in the periodic table?
... properties of that element. For example, an atom of carbon is represented in Figure 2.15. Each atom has a tiny, dense nucleus containing neutrons and protons. (A hydrogen nucleus has a single proton only.) The nucleus is surrounded by electrons, which exist in specific electron energy shells. Most o ...
... properties of that element. For example, an atom of carbon is represented in Figure 2.15. Each atom has a tiny, dense nucleus containing neutrons and protons. (A hydrogen nucleus has a single proton only.) The nucleus is surrounded by electrons, which exist in specific electron energy shells. Most o ...
Periodic Trends_CP_2016_Notes
... • ______ on the periodic table • Represent the ________ that the valence electrons occupy. • Valence Electrons – ____________ electrons in an atom. – The electrons that are lost, gained, or shared in chemical reactions. – The _________ ________ ________ in an element's outer shell determines the che ...
... • ______ on the periodic table • Represent the ________ that the valence electrons occupy. • Valence Electrons – ____________ electrons in an atom. – The electrons that are lost, gained, or shared in chemical reactions. – The _________ ________ ________ in an element's outer shell determines the che ...
CH 7 Peroidic trends
... These outer shell electrons are called valance electrons and are said to be in the valance shell. Therefore, there is a major difference in the chemical and physical properties between elements in different representative families, since each family differs in the number of outer shell electrons. Th ...
... These outer shell electrons are called valance electrons and are said to be in the valance shell. Therefore, there is a major difference in the chemical and physical properties between elements in different representative families, since each family differs in the number of outer shell electrons. Th ...
Document
... Read the IUPAC announcement about the names of the 4 new elements that will complete the 7th period on the periodic table. Write in those symbols on the periodic table I’ve given you which has blank spaces for these elements. Do additional research to find any remaining element symbols and names - a ...
... Read the IUPAC announcement about the names of the 4 new elements that will complete the 7th period on the periodic table. Write in those symbols on the periodic table I’ve given you which has blank spaces for these elements. Do additional research to find any remaining element symbols and names - a ...
Periodic Trends
... Periodic Trends Remember from the "Periodic Table" Notes... • The periodic table is a tabular display of the chemical elements, organized by their atomic number, electron configuration, and recurring properties. • Periodic law: There is a periodic repetition of chemical and physical properties of th ...
... Periodic Trends Remember from the "Periodic Table" Notes... • The periodic table is a tabular display of the chemical elements, organized by their atomic number, electron configuration, and recurring properties. • Periodic law: There is a periodic repetition of chemical and physical properties of th ...
Chem 100 unit 2
... I. COMPOUNDS - Two or more elements chemically combined in definite proportions. ...
... I. COMPOUNDS - Two or more elements chemically combined in definite proportions. ...
chapter 7- periodic properties of the elements
... created a set of cards of the elements- one card for each element currently discovered. • Each element card had information on it: the element’s name, atomic mass, properties, and compounds they formed with other elements ...
... created a set of cards of the elements- one card for each element currently discovered. • Each element card had information on it: the element’s name, atomic mass, properties, and compounds they formed with other elements ...
Periodic Trends
... For each of the four trends (atomic radius, ionization energy, reactivity, electronegativity) You need to know: Definitions of each trend Pattern of the trend (where on the periodic table is this trend the highest? The lowest?) Compare elements using trends (of these two elements, which has th ...
... For each of the four trends (atomic radius, ionization energy, reactivity, electronegativity) You need to know: Definitions of each trend Pattern of the trend (where on the periodic table is this trend the highest? The lowest?) Compare elements using trends (of these two elements, which has th ...
Name
... 2. He rearranged some elements out of atomic mass _______________ to keep them together with other elements with similar _____________________. He also left three ________________ in his table and correctly __________________ the properties of these 3 unidentified elements that were later identifie ...
... 2. He rearranged some elements out of atomic mass _______________ to keep them together with other elements with similar _____________________. He also left three ________________ in his table and correctly __________________ the properties of these 3 unidentified elements that were later identifie ...
NC SCS Chemistry
... guidance throughout the activity. The student instructions are listed below and correspond to the student handout. The bullets are instructions for the teacher and should be used to guide the activity. Using only the data on the element cards, identify patterns based on the properties. Put the eleme ...
... guidance throughout the activity. The student instructions are listed below and correspond to the student handout. The bullets are instructions for the teacher and should be used to guide the activity. Using only the data on the element cards, identify patterns based on the properties. Put the eleme ...