Chapter 8 notes
... • Because of penetration, sublevels of each energy level are NOT degenerate for multi-electron atoms • In fourth and fifth energy levels, penetration becomes so important that it results in 4s being lower energy than 3d • There can be variances in electron configuration, especially with TRANSITION M ...
... • Because of penetration, sublevels of each energy level are NOT degenerate for multi-electron atoms • In fourth and fifth energy levels, penetration becomes so important that it results in 4s being lower energy than 3d • There can be variances in electron configuration, especially with TRANSITION M ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... 3- he corrected the wrong estimated atomic weights of some elements. Why did he put more than an element in one place??? ...
... 3- he corrected the wrong estimated atomic weights of some elements. Why did he put more than an element in one place??? ...
10C The Periodic Table
... 6. What does the atomic number tell you about an element? Symbol and atomic mass: For each of the following, write the element name that corresponds to the symbol. In addition, write the atomic mass for each element. 7. Fe ...
... 6. What does the atomic number tell you about an element? Symbol and atomic mass: For each of the following, write the element name that corresponds to the symbol. In addition, write the atomic mass for each element. 7. Fe ...
Honors Chemistry: Chapter 6 Review
... 2) All elements can be divided into 3 categories: metals; nonmetals; metalloids 3) What type of elements are located to the left of the “staircase”? List any exceptions: metals; Hydrogen is a non-metal 4) What type of elements are located to the right of the “staircase”? List any exceptions: non-met ...
... 2) All elements can be divided into 3 categories: metals; nonmetals; metalloids 3) What type of elements are located to the left of the “staircase”? List any exceptions: metals; Hydrogen is a non-metal 4) What type of elements are located to the right of the “staircase”? List any exceptions: non-met ...
InterpretatingGraphics
... Thousands of periodic tables have been published since Mendeleev published his table. Each one is a little different from the rest. Shown above are keys to two of the hundreds of periodic tables now available. A key is an example or roadmap for using a periodic table. Use the above keys to answer th ...
... Thousands of periodic tables have been published since Mendeleev published his table. Each one is a little different from the rest. Shown above are keys to two of the hundreds of periodic tables now available. A key is an example or roadmap for using a periodic table. Use the above keys to answer th ...
History of the Periodic Table Chapter 6.1 Who developed the first
... advised to revise the periodic table. • After examining the properties of the elements, Seaborg was convinced that the elements were part of the inner transition elements because they behaved similarly to other transition metals. • Instead of creating a new system, he integrated the elements into a ...
... advised to revise the periodic table. • After examining the properties of the elements, Seaborg was convinced that the elements were part of the inner transition elements because they behaved similarly to other transition metals. • Instead of creating a new system, he integrated the elements into a ...
1 Periodic table and atomic structure
... they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
... they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
1 Periodic table and atomic structure
... they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
... they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
Addrienne`s Element Lesson Plan
... Definition: A row of the periodic table; each row corresponds to the number of electron shells in an atom of the elements in that row Context: The elements in the second period each have two electron shells, and the elements in the sixth period have six electron shells. periodic table of the element ...
... Definition: A row of the periodic table; each row corresponds to the number of electron shells in an atom of the elements in that row Context: The elements in the second period each have two electron shells, and the elements in the sixth period have six electron shells. periodic table of the element ...
Lecture 14-15 - U of L Class Index
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
to Ch 05 Periodic Trends
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
Unit Six: Atomic structure
... Copper, Cu, is a relatively soft metal, and a very good electrical conductor. ...
... Copper, Cu, is a relatively soft metal, and a very good electrical conductor. ...
Ch 4-1 Notes
... Group 2 = alkaline earth metals * each element has two electrons in an s sublevel * tend to lose two electrons to form a +2 ion and achieve an octet. * harder, denser, and stronger than alkali metals * have higher melting points than alkali metals * less reactive than alkali metals * not found in na ...
... Group 2 = alkaline earth metals * each element has two electrons in an s sublevel * tend to lose two electrons to form a +2 ion and achieve an octet. * harder, denser, and stronger than alkali metals * have higher melting points than alkali metals * less reactive than alkali metals * not found in na ...
Chapter 5 ppt
... • In their pure state, all of the alkali metals have a silvery appearance and are soft enough to cut with a knife. ...
... • In their pure state, all of the alkali metals have a silvery appearance and are soft enough to cut with a knife. ...
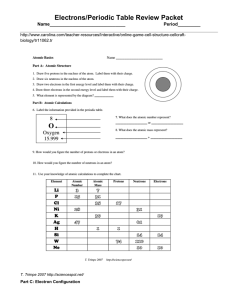
Electrons/Periodic Table Review Packet Name______________________________ Period_________
... 4. The element that has the atomic number 17 is? 5. List the symbols for two transition metals. 6. Cu, Ag, and Au are all in what group # 7. Name two noble gases 8. Give the symbol for two halogens. 9. What is the symbol for element with atomic number 74? 10. What is the atomic mass of copper? 11. W ...
... 4. The element that has the atomic number 17 is? 5. List the symbols for two transition metals. 6. Cu, Ag, and Au are all in what group # 7. Name two noble gases 8. Give the symbol for two halogens. 9. What is the symbol for element with atomic number 74? 10. What is the atomic mass of copper? 11. W ...
File
... Figure 2.32 The card above shows modern values for silicon, rather than the ones Mendeleev actually used. His values were surprisingly close to modern ones. The atomic mass measurement indicates that silicon is 28.1 times heavier than hydrogen. You can observe other properties of silicon in Figure 2 ...
... Figure 2.32 The card above shows modern values for silicon, rather than the ones Mendeleev actually used. His values were surprisingly close to modern ones. The atomic mass measurement indicates that silicon is 28.1 times heavier than hydrogen. You can observe other properties of silicon in Figure 2 ...
Topic 5 - Holy Cross Collegiate
... Figure 2.32 The card above shows modern values for silicon, rather than the ones Mendeleev actually used. His values were surprisingly close to modern ones. The atomic mass measurement indicates that silicon is 28.1 times heavier than hydrogen. You can observe other properties of silicon in Figure 2 ...
... Figure 2.32 The card above shows modern values for silicon, rather than the ones Mendeleev actually used. His values were surprisingly close to modern ones. The atomic mass measurement indicates that silicon is 28.1 times heavier than hydrogen. You can observe other properties of silicon in Figure 2 ...
Chapter 13
... Periodic Trend – There is a decrease in the size of cations as you move across a period from left to right – when you get to group 4A the anions (which are much larger) start to decrease in size Group Trend – Ionic size (both cations and anions) increases as you go down each group. ...
... Periodic Trend – There is a decrease in the size of cations as you move across a period from left to right – when you get to group 4A the anions (which are much larger) start to decrease in size Group Trend – Ionic size (both cations and anions) increases as you go down each group. ...
Student Exploration: Electron Configuration
... 6. Infer: Select the PERIODIC TABLE tab. The middle section of the table is a chemical family called the transition metals. Why do you think this section is ten columns wide? _________________________________________________________________________ ___________________________________________________ ...
... 6. Infer: Select the PERIODIC TABLE tab. The middle section of the table is a chemical family called the transition metals. Why do you think this section is ten columns wide? _________________________________________________________________________ ___________________________________________________ ...
Graphing Trends in the Periodic Table
... number. On the same graph, use a different color to do the same for elements in Family HA. Label the graph. 3. For elements 3—20, make a graph of the energy required to remove the easiest electron as a function of atomic number. Plot atomic number on the X axis and energy required on the Y axis. Lab ...
... number. On the same graph, use a different color to do the same for elements in Family HA. Label the graph. 3. For elements 3—20, make a graph of the energy required to remove the easiest electron as a function of atomic number. Plot atomic number on the X axis and energy required on the Y axis. Lab ...
Lectures 14-15 - U of L Class Index
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Zeff). The foll ...
The Atom Hypothesis
... 1.The elements, if arranged according to their atomic weights, exhibit an apparent periodicity of properties. 2.Elements which are similar as regards their chemical properties have atomic weights which are either of nearly the same value (eg. Pt, Ir, Os) or which increase regularly (eg. K, Ru, Cs). ...
... 1.The elements, if arranged according to their atomic weights, exhibit an apparent periodicity of properties. 2.Elements which are similar as regards their chemical properties have atomic weights which are either of nearly the same value (eg. Pt, Ir, Os) or which increase regularly (eg. K, Ru, Cs). ...
Chemistry Online Textbook
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.