Periodic Trends Periodic Table Development 1869
... o From left to right on the table, shielding is constant (electrons are being added to the same principal energy level- no new levels are added) o From top to bottom on the table, shielding increases (more principal energy levels are being added) Nuclear charge = pull or attraction for electrons b ...
... o From left to right on the table, shielding is constant (electrons are being added to the same principal energy level- no new levels are added) o From top to bottom on the table, shielding increases (more principal energy levels are being added) Nuclear charge = pull or attraction for electrons b ...
The Modern Periodic Table
... two reasons: 1.The loss of a valence electron can leave an empty outer orbital resulting in a small radius. 2.Electrostatic repulsion decreases allowing the electrons to be pulled closer to the radius. ...
... two reasons: 1.The loss of a valence electron can leave an empty outer orbital resulting in a small radius. 2.Electrostatic repulsion decreases allowing the electrons to be pulled closer to the radius. ...
to chapter:3
... discovered radium and polonium. They discovered that beta particles were negatively charged. In 1894 Sir William Ramsay and Lord Rayleigh discovered the noble gases, which were added to the periodic table as group 0. In 1897 English physicist J. J. Thomson first discovered electrons; small negativel ...
... discovered radium and polonium. They discovered that beta particles were negatively charged. In 1894 Sir William Ramsay and Lord Rayleigh discovered the noble gases, which were added to the periodic table as group 0. In 1897 English physicist J. J. Thomson first discovered electrons; small negativel ...
radiopharmaceutics l..
... electronic structure of each atom in a chemical bond containing 8 electrons. ● An octet in the outer shell makes atoms stable. ● Electrons are lost, gained or shared to form an octet. ● Metals lose electrons to match the number of valence electrons of their nearest noble gas e.g. He [2], neon [2,8] ...
... electronic structure of each atom in a chemical bond containing 8 electrons. ● An octet in the outer shell makes atoms stable. ● Electrons are lost, gained or shared to form an octet. ● Metals lose electrons to match the number of valence electrons of their nearest noble gas e.g. He [2], neon [2,8] ...
Atomic Structure and the Periodic Table of Elements: The Secret
... trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are put in order in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights. P ...
... trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are put in order in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights. P ...
Chapter 5- The Periodic Law
... 2. Along with the s-block are called the main-group elements 3. Properties vary greatly because some p-block elements are metals, some are non-metals and some are metalloids 4. The elements of Group 17 are known as the halogens. A. React with most metals to form salts B. F and Cl are gases at room t ...
... 2. Along with the s-block are called the main-group elements 3. Properties vary greatly because some p-block elements are metals, some are non-metals and some are metalloids 4. The elements of Group 17 are known as the halogens. A. React with most metals to form salts B. F and Cl are gases at room t ...
Period Trend
... Noble gases are unusual in that some of them do not form compounds and cannot be assigned electronegativity values ...
... Noble gases are unusual in that some of them do not form compounds and cannot be assigned electronegativity values ...
File - Mr. Walsh`s AP Chemistry
... by the valence shell electron(s) of Ar. This difference best describes which of the following? Na has________ ...
... by the valence shell electron(s) of Ar. This difference best describes which of the following? Na has________ ...
Chapter 5.1 History of PT - Effingham County Schools
... What is the trend of electronegativity? ...
... What is the trend of electronegativity? ...
Name
... 23. Circle the letter of each statement that is true about electronegativity values. a. The electronegativity values of the transition elements are all zero. b. The element with the highest electronegativity value is sodium. c. Nonmetals have higher electronegativity values than metals. d. Electrone ...
... 23. Circle the letter of each statement that is true about electronegativity values. a. The electronegativity values of the transition elements are all zero. b. The element with the highest electronegativity value is sodium. c. Nonmetals have higher electronegativity values than metals. d. Electrone ...
Lesson 4 History atom File
... 1. All matter is composed of atoms. The billiard-ball model of the atom (i.e., all atoms are tiny spheres). 2. All atoms of a given element are identical; atoms of different elements have different properties. Orally: Dalton characterized elements according to their atomic weight; however, when isot ...
... 1. All matter is composed of atoms. The billiard-ball model of the atom (i.e., all atoms are tiny spheres). 2. All atoms of a given element are identical; atoms of different elements have different properties. Orally: Dalton characterized elements according to their atomic weight; however, when isot ...
Chapter 8 Periodic Relationships Among the Elements Section 8.1
... Your author uses this section to introduce your students to the correct method of writing electron configurations for anions and cations. Electron configurations for representative elements are straight forward as long as your students understand the concept of isoelectronic (having the same number ...
... Your author uses this section to introduce your students to the correct method of writing electron configurations for anions and cations. Electron configurations for representative elements are straight forward as long as your students understand the concept of isoelectronic (having the same number ...
Trends of the Periodic Table
... Why? Elements on the right of the chart want to take others atom's electron (not given them up) because they are close to achieving the octet. The means it will require more energy to remove the outer most electron. Elements on the left of the chart would prefer to give up their electrons so it is e ...
... Why? Elements on the right of the chart want to take others atom's electron (not given them up) because they are close to achieving the octet. The means it will require more energy to remove the outer most electron. Elements on the left of the chart would prefer to give up their electrons so it is e ...
Regents Chemistry NOTE PACKET
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
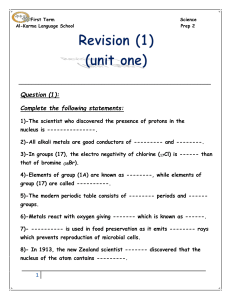
First Term Science Al-Karma Language School Prep 2 Question (1
... 6)-Metals react with oxygen giving ------- which is known as ------. 7)- ---------- is used in food preservation as it emits -------- rays which prevents reproduction of microbial cells. 8)- In 1913, the new Zealand scientist ------- discovered that the nucleus of the atom contains ---------. ...
... 6)-Metals react with oxygen giving ------- which is known as ------. 7)- ---------- is used in food preservation as it emits -------- rays which prevents reproduction of microbial cells. 8)- In 1913, the new Zealand scientist ------- discovered that the nucleus of the atom contains ---------. ...
File - pic sciences
... knock out an electron from the outer shell of the electron. Variation of ionization Energy in periods: In a period from left to right ionization energy do not follow any regular trend. In the first period ionization energy increases from hydrogen to helium. In the second period ionization potential ...
... knock out an electron from the outer shell of the electron. Variation of ionization Energy in periods: In a period from left to right ionization energy do not follow any regular trend. In the first period ionization energy increases from hydrogen to helium. In the second period ionization potential ...
Chapter #5 Notes
... • Main-group elements are the p-block and s-block elements. • Group 13- Boron's Family • Group 14- Carbon’s Family • Group 15- Nitrogen's Family • Group 16- Oxygen’s Family • Group 17- Halogens the most reactive nonmetals they form “salts” • Group 18- Noble Gases- Least reactive family. WHY???? ...
... • Main-group elements are the p-block and s-block elements. • Group 13- Boron's Family • Group 14- Carbon’s Family • Group 15- Nitrogen's Family • Group 16- Oxygen’s Family • Group 17- Halogens the most reactive nonmetals they form “salts” • Group 18- Noble Gases- Least reactive family. WHY???? ...
Chapter 6 - The Periodic Table
... For a period (row), the number of shielding electrons remain the same, but the number of protons in the nucleus increases. Example: All elements in the second period have the same underlying [He] noble gas configuration. However, the number of protons increase from left to right. ...
... For a period (row), the number of shielding electrons remain the same, but the number of protons in the nucleus increases. Example: All elements in the second period have the same underlying [He] noble gas configuration. However, the number of protons increase from left to right. ...
Chemistry Primer for pH Measurements
... In molecules, atoms are held together by sharing electrons (covalent bonds). In order to maximize these bonds, the atoms adopt specific positions relative to each other, i.e. each molecule has its own definite geometric structure. For instance in the water molecule, the two hydrogen atoms are bonded ...
... In molecules, atoms are held together by sharing electrons (covalent bonds). In order to maximize these bonds, the atoms adopt specific positions relative to each other, i.e. each molecule has its own definite geometric structure. For instance in the water molecule, the two hydrogen atoms are bonded ...
The Periodic Table Why is it called a periodic table?
... • Elements in the same group have the same number of valence electrons. • Number of groups in a block (s, p, d, f) corresponds to the maximum number of electrons that can occupy that sublevel. • 18 labeled groups ...
... • Elements in the same group have the same number of valence electrons. • Number of groups in a block (s, p, d, f) corresponds to the maximum number of electrons that can occupy that sublevel. • 18 labeled groups ...
Periodic Trends.notebook
... heat up. The temperature required to overcome the electrical barrier of 6 positive charges and the fusion of carbon nuclei is not reached. Thus, the carbon core contracts to an extremely dense white dwarf which radiates its last energy and becomes a black dwarf (which is really a giant diamond ...
... heat up. The temperature required to overcome the electrical barrier of 6 positive charges and the fusion of carbon nuclei is not reached. Thus, the carbon core contracts to an extremely dense white dwarf which radiates its last energy and becomes a black dwarf (which is really a giant diamond ...
Graphing Periodic Trends – Ana Julia Silva
... and crashing down to zero. 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. As you move across the periodic table, the number of protons increases by one. This affects ...
... and crashing down to zero. 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. As you move across the periodic table, the number of protons increases by one. This affects ...
(halogens group) 4-write down the electronic configuration
... 4-The density of pure water in solid state is………... a-less than its density in liquid state b- equal to its density in liquid state c- equal to its density in gaseous state d- greater than its density in liquid state 5-The density of pure water in solid state is ……… liquid state a-less than b- equal ...
... 4-The density of pure water in solid state is………... a-less than its density in liquid state b- equal to its density in liquid state c- equal to its density in gaseous state d- greater than its density in liquid state 5-The density of pure water in solid state is ……… liquid state a-less than b- equal ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.