History of the Periodic Table Chapter 6.1 Who developed the first
... advised to revise the periodic table. • After examining the properties of the elements, Seaborg was convinced that the elements were part of the inner transition elements because they behaved similarly to other transition metals. • Instead of creating a new system, he integrated the elements into a ...
... advised to revise the periodic table. • After examining the properties of the elements, Seaborg was convinced that the elements were part of the inner transition elements because they behaved similarly to other transition metals. • Instead of creating a new system, he integrated the elements into a ...
General Chemistry
... electronegativity increases from left to right across a period.electronegativity decreases down a group.small atoms with many protons in the nucleus have high electronegativity.the greater the difference in electronegativity of the two atoms, bond will be more polar. The most electronegative element ...
... electronegativity increases from left to right across a period.electronegativity decreases down a group.small atoms with many protons in the nucleus have high electronegativity.the greater the difference in electronegativity of the two atoms, bond will be more polar. The most electronegative element ...
III. Periodic Trends
... Reactive (not as reactive as Groups 1 or 2), can be free elements Highest melting points Electron Configuration ns2(n-1)dx where x is column in d-block Form variable valence state ions Always form Cations Examples: Co, Fe, Pt, etc ...
... Reactive (not as reactive as Groups 1 or 2), can be free elements Highest melting points Electron Configuration ns2(n-1)dx where x is column in d-block Form variable valence state ions Always form Cations Examples: Co, Fe, Pt, etc ...
Atomic Radius
... ability to attract electrons i.e. How badly does the atom want to gain electrons - Electronegativity is expressed as just a number (like “on a scale of 0-4”) ...
... ability to attract electrons i.e. How badly does the atom want to gain electrons - Electronegativity is expressed as just a number (like “on a scale of 0-4”) ...
Practice Packet Unit: 5 Periodic Table
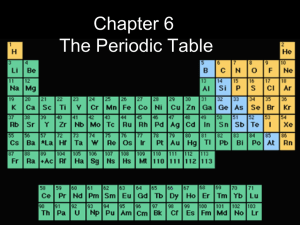
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
Chapter 5 – The Periodic Law
... electrons are in the highest (or outer) energy level • 5. The period number tells how many energy levels the atom has • a. The 1st period has only one sublevel (s), which can hold only 2 electrons; therefore, this period can have only 2 elements • b. The 2nd period has two sublevels (s & p, and p ha ...
... electrons are in the highest (or outer) energy level • 5. The period number tells how many energy levels the atom has • a. The 1st period has only one sublevel (s), which can hold only 2 electrons; therefore, this period can have only 2 elements • b. The 2nd period has two sublevels (s & p, and p ha ...
Summary of things you need to know from this first quarter. The
... • Some ‘groups’ contained elements with obviously differing properties (e.g. the group containing oxygen and sulfur also contained iron!) Of course, at the time, scientists did not know about protons and electrons (and hence atomic number) and many elements were, as yet undiscovered. ...
... • Some ‘groups’ contained elements with obviously differing properties (e.g. the group containing oxygen and sulfur also contained iron!) Of course, at the time, scientists did not know about protons and electrons (and hence atomic number) and many elements were, as yet undiscovered. ...
Elements and Atoms: The Building Blocks of Matter
... two-letter symbol; you will become familiar with some of these during this course. All the elements in your body are derived from the foods you eat and the air you breathe. ...
... two-letter symbol; you will become familiar with some of these during this course. All the elements in your body are derived from the foods you eat and the air you breathe. ...
GENERAL CHARACTERISTICS OF THE p
... these elements in nature is also far from any uniform pattern. Some of them are quite abundant, e.g., oxygen, silicon, aluminium, nitrogen etc. On the other hand the heavier members in each group of the block are generally much less abundant. The important minerals associated with elements will be c ...
... these elements in nature is also far from any uniform pattern. Some of them are quite abundant, e.g., oxygen, silicon, aluminium, nitrogen etc. On the other hand the heavier members in each group of the block are generally much less abundant. The important minerals associated with elements will be c ...
Intro
... we would expect polarizability to increase down a group as both the size and number of electrons increase. Conductivity. Metals are characterized by their abilities to conduct heat and electricity. As such, we can expect the trends in conductivity to mirror those of metallic character as well as ion ...
... we would expect polarizability to increase down a group as both the size and number of electrons increase. Conductivity. Metals are characterized by their abilities to conduct heat and electricity. As such, we can expect the trends in conductivity to mirror those of metallic character as well as ion ...
chemical periodicity
... s-Block: Outermost electrons are in the s-sublevel Alkali Metals – very active metals Alkaline Earth Metals – also active Hydrogen and Helium p-Block Groups 13 – 18 Electrons add to a p-sublevel only after the s- sublevel in the same energy level is filled Boron, carbon, nitrogen, oxygen, halogens,n ...
... s-Block: Outermost electrons are in the s-sublevel Alkali Metals – very active metals Alkaline Earth Metals – also active Hydrogen and Helium p-Block Groups 13 – 18 Electrons add to a p-sublevel only after the s- sublevel in the same energy level is filled Boron, carbon, nitrogen, oxygen, halogens,n ...
Atoms and The Periodic Table
... element, on being struck by cathode rays, emitted their own characteristic X-rays. This led Moseley to make a systematic study of the elements using X-rays. Moseley observed that as he went across a row of elements the frequency of the X-rays emitted became progressively higher as the atomic mass in ...
... element, on being struck by cathode rays, emitted their own characteristic X-rays. This led Moseley to make a systematic study of the elements using X-rays. Moseley observed that as he went across a row of elements the frequency of the X-rays emitted became progressively higher as the atomic mass in ...
January Exam Review
... 2 or more nonmetal atoms which act like ionic bonds and become charged 3. Mrs. Turriff and Mrs. Scandar have had enough of Robert the lab tech’s foul mood. They decided to sneak into the school in the middle of the night and mix all his chemicals. Surprisingly, some of the mixtures actually formed p ...
... 2 or more nonmetal atoms which act like ionic bonds and become charged 3. Mrs. Turriff and Mrs. Scandar have had enough of Robert the lab tech’s foul mood. They decided to sneak into the school in the middle of the night and mix all his chemicals. Surprisingly, some of the mixtures actually formed p ...
d) Ramsay. The idea of arranging the elements in the periodic table
... The ionization energies for removing successive electrons from sodium are 496 kJ/mol, 4562 kJ/mol, 6912 kJ/mol, and 9544 kJ/mol. The great jump in ionization energy after the first electron is removed indicates that a) sodium has four or five electrons. ...
... The ionization energies for removing successive electrons from sodium are 496 kJ/mol, 4562 kJ/mol, 6912 kJ/mol, and 9544 kJ/mol. The great jump in ionization energy after the first electron is removed indicates that a) sodium has four or five electrons. ...
Record: 1 THE EVOLUTION OF THE PERIODIC SYSTEM Page 1 of
... chemistry, Dimitri Ivanovich Mendeleev, completed the first of his numerous periodic charts. It included 63 known elements arranged according to increasing atomic weight; Mendeleev also left spaces for as yet undiscovered elements for which he predicted atomic weights. Prior to Mendeleev's discovery ...
... chemistry, Dimitri Ivanovich Mendeleev, completed the first of his numerous periodic charts. It included 63 known elements arranged according to increasing atomic weight; Mendeleev also left spaces for as yet undiscovered elements for which he predicted atomic weights. Prior to Mendeleev's discovery ...
The Periodic Table
... 21. Matter is made up of different kinds of particles called elements. 22. This copper wire, for example, is made up mostly of the element copper. 23. And, this substance is made up of the element mercury. 24. An element is the simplest type of a pure substance. . . 25. . . . which cannot be chemica ...
... 21. Matter is made up of different kinds of particles called elements. 22. This copper wire, for example, is made up mostly of the element copper. 23. And, this substance is made up of the element mercury. 24. An element is the simplest type of a pure substance. . . 25. . . . which cannot be chemica ...
Chapter 7 - HCC Learning Web
... • Elements in the same column contain the same number of outer-shell electrons or valence electrons. • How do we organize the different elements in a meaningful way that will allow us to make predictions about undiscovered elements? • Arrange elements to reflect the trends in chemical and physical p ...
... • Elements in the same column contain the same number of outer-shell electrons or valence electrons. • How do we organize the different elements in a meaningful way that will allow us to make predictions about undiscovered elements? • Arrange elements to reflect the trends in chemical and physical p ...
The Structure of the Atom and the Periodic Table
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s, the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electromagnetic spectru ...
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s, the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electromagnetic spectru ...
03 Chapter 2 Atomic Structure Power point Periodic Table
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
CHAPTER – 8 THE d- and f- BLOCK ELEMENTS
... The very name ‘transition’ given to the elements of d-block is only because of their position between s– and p– block elements. The d–orbitals of the penultimate energy level in their atoms receive electrons giving rise to the three rows of the transition metals, i.e., 3d, 4d and 5d. The fourth row ...
... The very name ‘transition’ given to the elements of d-block is only because of their position between s– and p– block elements. The d–orbitals of the penultimate energy level in their atoms receive electrons giving rise to the three rows of the transition metals, i.e., 3d, 4d and 5d. The fourth row ...
How can atomic theory explain patterns in the periodic table?
... elements, because they all have full valence shells. This feature makes them unusually stable. Their atoms do not tend to gain, lose, or share electrons with other elements— for the most part, they are unreactive. As you can see in Figure 2.18, helium has two electrons, which is the maximum number o ...
... elements, because they all have full valence shells. This feature makes them unusually stable. Their atoms do not tend to gain, lose, or share electrons with other elements— for the most part, they are unreactive. As you can see in Figure 2.18, helium has two electrons, which is the maximum number o ...
The Atom Hypothesis
... 2.Elements which are similar as regards their chemical properties have atomic weights which are either of nearly the same value (eg. Pt, Ir, Os) or which increase regularly (eg. K, Ru, Cs). 3.The arrangement of the elements, or of groups of elements in the order of their atomic weights, corresponds ...
... 2.Elements which are similar as regards their chemical properties have atomic weights which are either of nearly the same value (eg. Pt, Ir, Os) or which increase regularly (eg. K, Ru, Cs). 3.The arrangement of the elements, or of groups of elements in the order of their atomic weights, corresponds ...
Chemistry 1 Chapter 4, The Periodic Table
... • group 1 – alkali metals, they have a single valence electrons and are very reactive • they are never found in nature as pure elements because they are so reactive they are always combined with other elements as compounds •group 2 – alkaline-earth metals, they have 2 valence electrons, they must lo ...
... • group 1 – alkali metals, they have a single valence electrons and are very reactive • they are never found in nature as pure elements because they are so reactive they are always combined with other elements as compounds •group 2 – alkaline-earth metals, they have 2 valence electrons, they must lo ...
Chapter 5: Electrons
... Describe the periodic tables of Moseley and Mendeleev. Identify the various families of elements on the periodic table. State the trends in atomic radius, ionization energy, electron affinity and ion size with a group or period on the periodic table. Identify the relationship between these t ...
... Describe the periodic tables of Moseley and Mendeleev. Identify the various families of elements on the periodic table. State the trends in atomic radius, ionization energy, electron affinity and ion size with a group or period on the periodic table. Identify the relationship between these t ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.