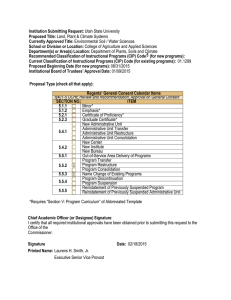
03/27/15 Approved - Utah State University
... PSC 13XX Forum on the Land, Plant, & Climate Systems (BPS) Introductory breadth physical science class designed to explore global challenges facing the world and local communities in food security, water availability, land degradation, climate change and agricultural and environmental sustainability ...
... PSC 13XX Forum on the Land, Plant, & Climate Systems (BPS) Introductory breadth physical science class designed to explore global challenges facing the world and local communities in food security, water availability, land degradation, climate change and agricultural and environmental sustainability ...
Thermal Decomposition of Polymers - Marcelo Hirschler
... complicated than that of flammable liquids. For most flammable liquids, the gasification process is simply evaporation. The liquid evaporates at a rate required to maintain the equilibrium vapor pressure above the liquid. In the case of polymeric materials, the original material itself is essentiall ...
... complicated than that of flammable liquids. For most flammable liquids, the gasification process is simply evaporation. The liquid evaporates at a rate required to maintain the equilibrium vapor pressure above the liquid. In the case of polymeric materials, the original material itself is essentiall ...
Cultivation of American ginseng
... ginseng, an endangered species. In Canada, it is against the law to harvest wild populations of American ginseng. This law does impact producers since they need to acquire a permit to export cultivated ginseng roots. Since the roots of the cultivated variety is identical to the wild variety, produce ...
... ginseng, an endangered species. In Canada, it is against the law to harvest wild populations of American ginseng. This law does impact producers since they need to acquire a permit to export cultivated ginseng roots. Since the roots of the cultivated variety is identical to the wild variety, produce ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... the combination of two different compounds to form a new compound. For example, oxygen reacts with sulfur dioxide to make sulfur trioxide. 2 SO2(g) + O2(g) 2 SO3(g) Sulfur trioxide and water undergo a combination reaction to form sulfuric acid. SO3(g) + H2O() H2SO4(aq) Notice that in all combin ...
... the combination of two different compounds to form a new compound. For example, oxygen reacts with sulfur dioxide to make sulfur trioxide. 2 SO2(g) + O2(g) 2 SO3(g) Sulfur trioxide and water undergo a combination reaction to form sulfuric acid. SO3(g) + H2O() H2SO4(aq) Notice that in all combin ...
Acids and Bases Unit
... This is a guessing question so be prepared for different responses. Most likely, that the light bulb will light up After testing the conductivity of the strong acid and before testing the weak acid in solution: o Did anything happen to the light bulb? Is that what we expected? If done correct ...
... This is a guessing question so be prepared for different responses. Most likely, that the light bulb will light up After testing the conductivity of the strong acid and before testing the weak acid in solution: o Did anything happen to the light bulb? Is that what we expected? If done correct ...
Using turnips to reduce soil K loading on the effluent block
... grass silage to remove K (Table 1) may avoid the need for excessively large land-treatment areas (Hedley et al. 2004). Turnips potentially produce larger amounts of dry matter with a higher metabolisable energy and K content (Table 1) than conserved pasture. For the same growing period, turnips at a ...
... grass silage to remove K (Table 1) may avoid the need for excessively large land-treatment areas (Hedley et al. 2004). Turnips potentially produce larger amounts of dry matter with a higher metabolisable energy and K content (Table 1) than conserved pasture. For the same growing period, turnips at a ...
RedOx notes:
... must have the charge that makes everything else sum to zero. If you don’t choose first you might not get your first choice (you can’t always get what you wanted, but if you try real hard you (might just get) what you (“We, the compound”) need) ...
... must have the charge that makes everything else sum to zero. If you don’t choose first you might not get your first choice (you can’t always get what you wanted, but if you try real hard you (might just get) what you (“We, the compound”) need) ...
Organic and Bio-Molecular Chemistry
... Francesco Nicotra Department of Biotechnology and Biosciences, University of Milano-Bicocca, Milano, ...
... Francesco Nicotra Department of Biotechnology and Biosciences, University of Milano-Bicocca, Milano, ...
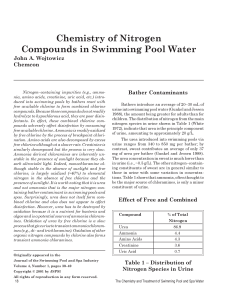
w_4-3 Chemistry of Nitrogen Compounds
... Nitrogen–containing impurities (e.g., ammonia, amino acids, creatinine, uric acid, etc.) introduced into swimming pools by bathers react with free available chlorine to form combined chlorine compounds. Because these compounds do not readily hydrolyze to hypochlorous acid, they are poor disinfectant ...
... Nitrogen–containing impurities (e.g., ammonia, amino acids, creatinine, uric acid, etc.) introduced into swimming pools by bathers react with free available chlorine to form combined chlorine compounds. Because these compounds do not readily hydrolyze to hypochlorous acid, they are poor disinfectant ...
Organic Production Systems Guidelines
... North America, is increasing for food and agricultural products that are perceived to be healthy and have low impact on the environment. A willingness to pay a premium for such products is apparent where products carry a verifiable assurance they are safe, nutritious and produced using systems with ...
... North America, is increasing for food and agricultural products that are perceived to be healthy and have low impact on the environment. A willingness to pay a premium for such products is apparent where products carry a verifiable assurance they are safe, nutritious and produced using systems with ...
Acids, Bases and Salts
... 4. What concentration results from mixing 400 mL of 2.0 M HCl with 600 mL of 3.0 M HCl? 5. What is the concentration of NaCl when 3 L of 0.5 M NaCl are mixed with 2 L of 0.2 M NaCl? 6. What is the concentration of NaCl when 3 L of 0.5 M NaCl are mixed with 2 L of water? 7. Water is added to 4 L of 6 ...
... 4. What concentration results from mixing 400 mL of 2.0 M HCl with 600 mL of 3.0 M HCl? 5. What is the concentration of NaCl when 3 L of 0.5 M NaCl are mixed with 2 L of 0.2 M NaCl? 6. What is the concentration of NaCl when 3 L of 0.5 M NaCl are mixed with 2 L of water? 7. Water is added to 4 L of 6 ...
Ni recovery using KOH, NaOH, and NH4OH in the presence of
... potassium hydroxide, showed that the stability zone of the jarosite is within pH 1 to 3 and temperatures between 20°C and 200°C; at low pH values no precipitate occurs, but at a high pH values mainly goethite (up to 100°C) and haematite (above 100°C) appear (Ismael and Carvalho, ...
... potassium hydroxide, showed that the stability zone of the jarosite is within pH 1 to 3 and temperatures between 20°C and 200°C; at low pH values no precipitate occurs, but at a high pH values mainly goethite (up to 100°C) and haematite (above 100°C) appear (Ismael and Carvalho, ...
The Effect of Water-Rock Interaction on
... 2.1. Groundwater in volcanic aquifers of Ethiopia ......................................................................5 2.2. Water-rock interaction and its control on chemical composition .........................................7 2.3. Aqueous speciation and solubility............................. ...
... 2.1. Groundwater in volcanic aquifers of Ethiopia ......................................................................5 2.2. Water-rock interaction and its control on chemical composition .........................................7 2.3. Aqueous speciation and solubility............................. ...
Heterogeneous Electron-Transfer Kinetics for
... appropriate length (HS(CH2)mCH3, where m ) n - 1). The total concentration of thiol in these coating solutions was approximately 1.0 × 10-3 M, and the mole fraction of the redox-active thiol was varied to give different concentrations of the redox couple in the monolayer. The electrodes remained in ...
... appropriate length (HS(CH2)mCH3, where m ) n - 1). The total concentration of thiol in these coating solutions was approximately 1.0 × 10-3 M, and the mole fraction of the redox-active thiol was varied to give different concentrations of the redox couple in the monolayer. The electrodes remained in ...
Chapter 7 - NordoniaHonorsChemistry
... The net ionic equation for an acid-base reaction often is: H+1(aq) + OH-1(aq) H2O(l) As long as the salt that forms is soluble in water. Tro's "Introductory Chemistry", Chapter 7 ...
... The net ionic equation for an acid-base reaction often is: H+1(aq) + OH-1(aq) H2O(l) As long as the salt that forms is soluble in water. Tro's "Introductory Chemistry", Chapter 7 ...
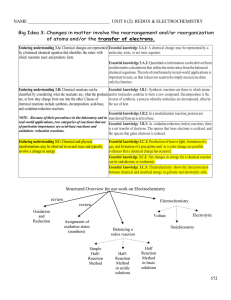
Oxidation numbers
... So the oxidation number of carbon is +4. The oxidation number of carbon in the products is 0 and in the reactants it is +4 The oxidation number has increased (become more positive). The oxidation number of oxygen in the products is 0 and in the reactants it is −2 The oxidation number has decreased ( ...
... So the oxidation number of carbon is +4. The oxidation number of carbon in the products is 0 and in the reactants it is +4 The oxidation number has increased (become more positive). The oxidation number of oxygen in the products is 0 and in the reactants it is −2 The oxidation number has decreased ( ...
VALLEY VIEW UNIVERSITY 2008 Fertilisation with human urine in a nutshell_0
... Urine is the fluid excreted by the kidneys. It consists of water, carrying in solution the body's waste products and excess substances. Most of the nutrients absorbed by the human body from the food we eat is excreted via urine. Therefore, urine is rich i ...
... Urine is the fluid excreted by the kidneys. It consists of water, carrying in solution the body's waste products and excess substances. Most of the nutrients absorbed by the human body from the food we eat is excreted via urine. Therefore, urine is rich i ...
Packet 1 - Kentucky Community and Technical College System
... This type of reaction can be called an exchange reaction, double displacement or metathesis. There are three basic types: a.) precipitation, b.) acid-base, and c.) gas-forming. All involve the exchange of ions to form a new . As my high school chemistry instructor liked to say, “Switch partners and ...
... This type of reaction can be called an exchange reaction, double displacement or metathesis. There are three basic types: a.) precipitation, b.) acid-base, and c.) gas-forming. All involve the exchange of ions to form a new . As my high school chemistry instructor liked to say, “Switch partners and ...
Document
... Rule #4: The oxidation number of oxygen in most of its compounds is -2. Exceptions: in peroxides, the oxidation number of oxygen is -1. in a binary compound with fluorine the oxidation number may be +1 or +2 Rule #5: The oxidation number of hydrogen in most of its compounds is +1, Exception: in hydr ...
... Rule #4: The oxidation number of oxygen in most of its compounds is -2. Exceptions: in peroxides, the oxidation number of oxygen is -1. in a binary compound with fluorine the oxidation number may be +1 or +2 Rule #5: The oxidation number of hydrogen in most of its compounds is +1, Exception: in hydr ...
Chem12 SM Unit 5 Review final ok
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
Origin and metamorphic evolution of magnesite
... Granite forms small bodies cropping out at several localities in the Gemericum. The presence of large volumes of granite at depth was confirmed by numerous boreholes and geophysical measurements (Plančar et al. 1977). In the Dlhá Dolina Valley, the granite is not exposed on the surface, but was pene ...
... Granite forms small bodies cropping out at several localities in the Gemericum. The presence of large volumes of granite at depth was confirmed by numerous boreholes and geophysical measurements (Plančar et al. 1977). In the Dlhá Dolina Valley, the granite is not exposed on the surface, but was pene ...
Document
... Rule #4: The oxidation number of oxygen in most of its compounds is -2. Exceptions: in peroxides, the oxidation number of oxygen is -1. in a binary compound with fluorine the oxidation number may be +1 or +2 Rule #5: The oxidation number of hydrogen in most of its compounds is +1, Exception: in hydr ...
... Rule #4: The oxidation number of oxygen in most of its compounds is -2. Exceptions: in peroxides, the oxidation number of oxygen is -1. in a binary compound with fluorine the oxidation number may be +1 or +2 Rule #5: The oxidation number of hydrogen in most of its compounds is +1, Exception: in hydr ...
Solutions_C19
... The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. This means nitrogen has 3 “extra” electrons that give it a – 3 oxidation number. The hydrogen atoms ...
... The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. This means nitrogen has 3 “extra” electrons that give it a – 3 oxidation number. The hydrogen atoms ...
Second Year - WordPress.com
... periodic function of their atomic mass. c) The x – rays spectra of the elements are more complex than the optical spectra. d) The properties of elements are the periodic function of their atomic number. ...
... periodic function of their atomic mass. c) The x – rays spectra of the elements are more complex than the optical spectra. d) The properties of elements are the periodic function of their atomic number. ...
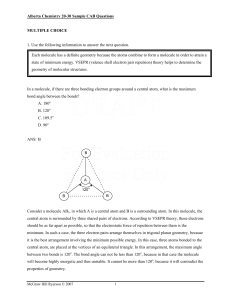
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...