Erosion and Sediment Control - International Erosion Control
... - adversely affect the health and biodiversity of aquatic life within these water bodies - increase the concentration of nutrients and metals within these waters - reduce light penetration into the water - increase the risk and cost of water treatment works associated with both farm and town water s ...
... - adversely affect the health and biodiversity of aquatic life within these water bodies - increase the concentration of nutrients and metals within these waters - reduce light penetration into the water - increase the risk and cost of water treatment works associated with both farm and town water s ...
The role of aqueous-phase oxidation in the A
... mate change, air quality, and human health. Our ability to predict its formation and fate is hindered by uncertainties associated with one type in particular, organic aerosol (OA). Ambient OA measurements indicate that it can become highly oxidized in short timescales, but this is generally not repr ...
... mate change, air quality, and human health. Our ability to predict its formation and fate is hindered by uncertainties associated with one type in particular, organic aerosol (OA). Ambient OA measurements indicate that it can become highly oxidized in short timescales, but this is generally not repr ...
Review of the Lithium Isotope System as a Geochemical Tracer
... is present only in the +1 valence state, so its isotopic composition is not influenced by redox reaction. During weathering, the lighter isotope 6Li is preferentially retained in the solid phase whilst 7Li goes into solution (Huh et al., 1998, 2001, 2004; Pistiner and Henderson, 2003; Kisakürek et a ...
... is present only in the +1 valence state, so its isotopic composition is not influenced by redox reaction. During weathering, the lighter isotope 6Li is preferentially retained in the solid phase whilst 7Li goes into solution (Huh et al., 1998, 2001, 2004; Pistiner and Henderson, 2003; Kisakürek et a ...
The Mantle and its Products
... considered an obstacle to slabs of oceanic lithosphere that had been subducted into the mantle, but evidence from seismic tomography (a technique in which seismic waves can be used to produce three-dimensional images of Earth’s interior) suggests that its role in acting as an impermeable barrier may ...
... considered an obstacle to slabs of oceanic lithosphere that had been subducted into the mantle, but evidence from seismic tomography (a technique in which seismic waves can be used to produce three-dimensional images of Earth’s interior) suggests that its role in acting as an impermeable barrier may ...
Chapter 9 Review, pages 628–633
... 25. (a) Separate the equation H2O(l) + Au3+(aq) → O2(g) + Au(s) into two half-reactions. H2O(l) → O2(g) (oxidation) Au3+(aq) → Au(s) (reduction) For the oxidation half-reaction, first balance oxygen. 2 H2O(l) → O2(g) Balance hydrogen by adding hydrogen ions. 2 H2O(l) → O2(g) + 4 H+(aq) Balance the c ...
... 25. (a) Separate the equation H2O(l) + Au3+(aq) → O2(g) + Au(s) into two half-reactions. H2O(l) → O2(g) (oxidation) Au3+(aq) → Au(s) (reduction) For the oxidation half-reaction, first balance oxygen. 2 H2O(l) → O2(g) Balance hydrogen by adding hydrogen ions. 2 H2O(l) → O2(g) + 4 H+(aq) Balance the c ...
Chemical Reactions - 2012 Book Archive
... chemical compound has a particular combination of atoms and that the ratios of the numbers of atoms of the elements present are usually small whole numbers. We also described the law of multiple proportions, which states that the ratios of the masses of elements that form a series of compounds are s ...
... chemical compound has a particular combination of atoms and that the ratios of the numbers of atoms of the elements present are usually small whole numbers. We also described the law of multiple proportions, which states that the ratios of the masses of elements that form a series of compounds are s ...
laman web smk raja perempuan, ipoh
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Acids and Bases
... a species that donates a proton, and a base is a species that accepts a proton. (Remember that positively charged hydrogen ions are called protons.) In the following reaction, hydrogen chloride (HCl) is an acid because it donates a proton to water, and water is a base because it accepts a proton fro ...
... a species that donates a proton, and a base is a species that accepts a proton. (Remember that positively charged hydrogen ions are called protons.) In the following reaction, hydrogen chloride (HCl) is an acid because it donates a proton to water, and water is a base because it accepts a proton fro ...
PACRIM 2015 Congress, Hong Kong, China, 18
... epidote- and actinolite-bearing and magnetite-rich high temperature propylitic zone. Minor relic hydrothermal biotite is identifiable in thin-section where it is pervasively overprinted by chlorite at deeper levels and by retrograde phyllic and intermediate argillic assemblages at shallower levels. ...
... epidote- and actinolite-bearing and magnetite-rich high temperature propylitic zone. Minor relic hydrothermal biotite is identifiable in thin-section where it is pervasively overprinted by chlorite at deeper levels and by retrograde phyllic and intermediate argillic assemblages at shallower levels. ...
aq - Haverford Alchemy
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Lithosphere and Asthenosphere
... about 100 kilometers thick. How are crust and lithosphere different from each other? ...
... about 100 kilometers thick. How are crust and lithosphere different from each other? ...
Photo-oxidation of pinonaldehyde at low NOx
... is relatively volatile and prone to fragmentation, it is in some sense a worst-case example of aging chemistry; if pinonaldehyde produces substantial SOA under both high- and lowNOx conditions, it is extremely likely that many other less volatile first-generation α-pinene oxidation products will be ...
... is relatively volatile and prone to fragmentation, it is in some sense a worst-case example of aging chemistry; if pinonaldehyde produces substantial SOA under both high- and lowNOx conditions, it is extremely likely that many other less volatile first-generation α-pinene oxidation products will be ...
Nitrogen and Oxygen Family
... Nitrates are all very soluble in water so they are not wide spread in the earth’s crust. NaNO3 is found together with small amounts of KNO3, CaSO4 and NaIO3 along the coast of southern Chile under a thin layer of sand or soil. Nitrates are difficult to reduce under the laboratory conditions but micr ...
... Nitrates are all very soluble in water so they are not wide spread in the earth’s crust. NaNO3 is found together with small amounts of KNO3, CaSO4 and NaIO3 along the coast of southern Chile under a thin layer of sand or soil. Nitrates are difficult to reduce under the laboratory conditions but micr ...
extra revision sheet grade 7 Q 4 Multiple Choice Identify the choice
... ____ 26. Ice, wind, water, gravity, plants, and animals are all agents of a. oxidation. c. differential weathering. b. desertification. d. physical weathering. ...
... ____ 26. Ice, wind, water, gravity, plants, and animals are all agents of a. oxidation. c. differential weathering. b. desertification. d. physical weathering. ...
Full Text
... layer of strength, the lithosphere must encompass the region where these factors (either singularly or in combination) work to promote strength. For example, the lithosphere is strong because it is at temperatures sufficiently cool enough to promote rigid behavior, or the lithosphere is strong not o ...
... layer of strength, the lithosphere must encompass the region where these factors (either singularly or in combination) work to promote strength. For example, the lithosphere is strong because it is at temperatures sufficiently cool enough to promote rigid behavior, or the lithosphere is strong not o ...
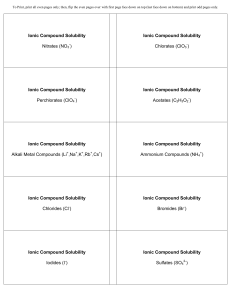
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... To Print, print all even pages only; then, flip the even pages over with first page face down on top (last face down on bottom) and print odd pages only. ...
... To Print, print all even pages only; then, flip the even pages over with first page face down on top (last face down on bottom) and print odd pages only. ...
Phacelia tanacetifolia: A brief overview of a potentially useful
... well in dry soil. It does a good job of limiting nitrate leaching when planted in early fall. It winterkills at about 18°F. In cooler regions, it can be used as a between cash crops cover crop in the summer. Phacelia is listed as one of the top 20 honey-producing flowers for honeybees and is also hi ...
... well in dry soil. It does a good job of limiting nitrate leaching when planted in early fall. It winterkills at about 18°F. In cooler regions, it can be used as a between cash crops cover crop in the summer. Phacelia is listed as one of the top 20 honey-producing flowers for honeybees and is also hi ...
Midwest Blueberry Production Guide
... Description: Oldest, largest fruit in clusters begin to change color from green to pink to blue. Fruit begins to soften. Cell division has stopped and fruit growth is by ...
... Description: Oldest, largest fruit in clusters begin to change color from green to pink to blue. Fruit begins to soften. Cell division has stopped and fruit growth is by ...
Optimizing Crop N Use Efficiency Using a Variable Source N
... efficiency (NUE) within agricultural fields is needed to improve crop production and reduce nitrogen (N) loss. Field studies planted to corn (Zea mays L.) were conducted in 2005 and 2006 in the claypan region of north central (Centralia) and northeast Missouri (Greenley Research Center) to determine ...
... efficiency (NUE) within agricultural fields is needed to improve crop production and reduce nitrogen (N) loss. Field studies planted to corn (Zea mays L.) were conducted in 2005 and 2006 in the claypan region of north central (Centralia) and northeast Missouri (Greenley Research Center) to determine ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • In the ionic equation all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) Aqueous Reactions AgCl (s) + K+ (aq) ...
... • In the ionic equation all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) Aqueous Reactions AgCl (s) + K+ (aq) ...
Lithosphere and Asthenosphere
... The definition of the lithosphere is based on how Earth materials behave, so it includes the crust and the uppermost mantle, which are both brittle. Since it is rigid and brittle, when stresses act on the lithosphere, it breaks. This is what we experience as an earthquake. Although we sometimes refe ...
... The definition of the lithosphere is based on how Earth materials behave, so it includes the crust and the uppermost mantle, which are both brittle. Since it is rigid and brittle, when stresses act on the lithosphere, it breaks. This is what we experience as an earthquake. Although we sometimes refe ...