14.1 The Atmosphere and Atmospheric chemistry
... Ammonia is particularly important as a base in the air because it is the only water-soluble base present at significant levels in the atmosphere. When it is dissolved in atmospheric water droplets, ammonia plays a strong role in neutralizing atmospheric acids, as shown by the following reactions: ...
... Ammonia is particularly important as a base in the air because it is the only water-soluble base present at significant levels in the atmosphere. When it is dissolved in atmospheric water droplets, ammonia plays a strong role in neutralizing atmospheric acids, as shown by the following reactions: ...
Lecture Notes
... *These rocks consist of various minerals which are called, Rock Forming Minerals. Some of them are useful for industry (eg. Feldspar Ceramics, and Glass industry, or Diatomite, a sedimentary rock Sugar industry) and are called Industrial Minerals and Rocks. Some minerals have high concentrations ...
... *These rocks consist of various minerals which are called, Rock Forming Minerals. Some of them are useful for industry (eg. Feldspar Ceramics, and Glass industry, or Diatomite, a sedimentary rock Sugar industry) and are called Industrial Minerals and Rocks. Some minerals have high concentrations ...
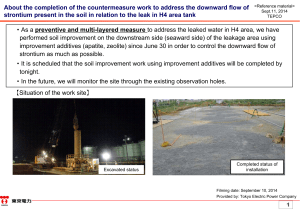
About the completion of the countermeasure work to address the
... From a result of desorption test after strontium absorption, the quantity of strontium incorporated into the structure of apatite is 8.5mg/g(corresponding to approximately 0.1mol (strontium) to 1mol (apatite)). In the leaked 300t of contaminated water, radioactive Sr and seawater origined stable Sr ...
... From a result of desorption test after strontium absorption, the quantity of strontium incorporated into the structure of apatite is 8.5mg/g(corresponding to approximately 0.1mol (strontium) to 1mol (apatite)). In the leaked 300t of contaminated water, radioactive Sr and seawater origined stable Sr ...
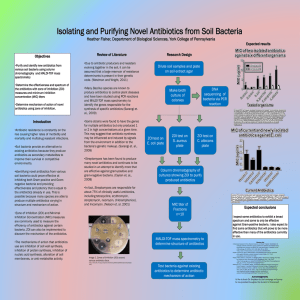
(MIC) titers Determine mechanism of action of novel antibiotics using
... Gram-negative bacteria, and a yeasts. Antibiotic A shows inhibition ofGram positive-bacteria, but not much effect on Gram-negative bacteria or yeast. Antibiotic B shows inhibition of all organisms. Antibiotic A is narrow spectrum, while Antibiotic B is broad ...
... Gram-negative bacteria, and a yeasts. Antibiotic A shows inhibition ofGram positive-bacteria, but not much effect on Gram-negative bacteria or yeast. Antibiotic B shows inhibition of all organisms. Antibiotic A is narrow spectrum, while Antibiotic B is broad ...
ConcepTest On Simple Redox Reactions
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
File
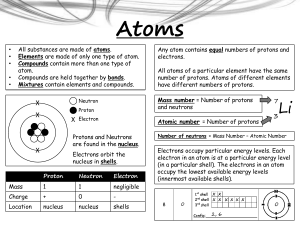
... s) Isoelectronic: atoms and ions that are isoelectronic have the same electron configuration. Usually atoms gain or lose electrons to form a stable octet electron arrangement, this makes the ions that form isoelectronic with Noble gases. t) Halogen: the Halogen Family is the common name for the Grou ...
... s) Isoelectronic: atoms and ions that are isoelectronic have the same electron configuration. Usually atoms gain or lose electrons to form a stable octet electron arrangement, this makes the ions that form isoelectronic with Noble gases. t) Halogen: the Halogen Family is the common name for the Grou ...
File - Mc Guckin Science
... s) Isoelectronic: atoms and ions that are isoelectronic have the same electron configuration. Usually atoms gain or lose electrons to form a stable octet electron arrangement, this makes the ions that form isoelectronic with Noble gases. t) Halogen: the Halogen Family is the common name for the Grou ...
... s) Isoelectronic: atoms and ions that are isoelectronic have the same electron configuration. Usually atoms gain or lose electrons to form a stable octet electron arrangement, this makes the ions that form isoelectronic with Noble gases. t) Halogen: the Halogen Family is the common name for the Grou ...
SAND - Soil Scientists Wiki
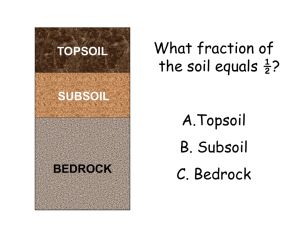
... field, and tomatoes in 1/8 of his field. Using the key, color his field to show these fractions. ...
... field, and tomatoes in 1/8 of his field. Using the key, color his field to show these fractions. ...
Chapter 2 - MrJardina
... Rock Gypsum- forms when H²O evaporates. Rock Salt- (almost pure halite) is known to you as table salt. ...
... Rock Gypsum- forms when H²O evaporates. Rock Salt- (almost pure halite) is known to you as table salt. ...
Download the Full Article
... Research conducted previously at Walpeup in the 1980s1 found that maintaining 2 t/ha stubble increased the amount of water stored in the soil at sowing, at depth 0-140 cm, by 16 mm in 1 year of 3. This increase in soil water availability was not reflected in increased crop yield. The recent experime ...
... Research conducted previously at Walpeup in the 1980s1 found that maintaining 2 t/ha stubble increased the amount of water stored in the soil at sowing, at depth 0-140 cm, by 16 mm in 1 year of 3. This increase in soil water availability was not reflected in increased crop yield. The recent experime ...
Training and development pack on turf diseases for staff
... Fungal spores can remain inactive in the environment for extremely long periods and become active again when conditions are suitable. This fact makes the control of fungal diseases extremely hard i.e. they is always a large deposit of potential agents of infection present (in the soil, plant debris ...
... Fungal spores can remain inactive in the environment for extremely long periods and become active again when conditions are suitable. This fact makes the control of fungal diseases extremely hard i.e. they is always a large deposit of potential agents of infection present (in the soil, plant debris ...
puckett attendance center
... 4b. Describe the cause and effect relationship between the composition of and movement within the Earth’s lithosphere. (DOK 1) •Seismic wave velocities of earthquakes and volcanoes to lithospheric plate boundaries using seismic data •Volcanoes formed at mid-ocean ridges, within intra-plate regions, ...
... 4b. Describe the cause and effect relationship between the composition of and movement within the Earth’s lithosphere. (DOK 1) •Seismic wave velocities of earthquakes and volcanoes to lithospheric plate boundaries using seismic data •Volcanoes formed at mid-ocean ridges, within intra-plate regions, ...
Chemistry
... Describe and apply the scientific method. Demonstrate critical thinking skills through qualitative and quantitative analyses tasks. Use dimensional analysis with proper attention to units and significant figures. Describe chemical and physical properties of matter. Explain the basic model of the ato ...
... Describe and apply the scientific method. Demonstrate critical thinking skills through qualitative and quantitative analyses tasks. Use dimensional analysis with proper attention to units and significant figures. Describe chemical and physical properties of matter. Explain the basic model of the ato ...
Ionic bonding
... Why electrolysis is expensive (2 reasons)? Name the process for taking ores out of the ground. ...
... Why electrolysis is expensive (2 reasons)? Name the process for taking ores out of the ground. ...
Redox
... The oxidation number is used to express the oxidation state of an element, whether as the uncombined element or when combined in a compound; it consists of a + or – sign followed by a number, or it is zero. Atoms of elements have no overall charge and are therefore given an oxidation number of zero. ...
... The oxidation number is used to express the oxidation state of an element, whether as the uncombined element or when combined in a compound; it consists of a + or – sign followed by a number, or it is zero. Atoms of elements have no overall charge and are therefore given an oxidation number of zero. ...
Ionic bonding
... What gas is released when carbonates are heated strongly? What solid product is formed when zinc carbonate is heated? What is the name of the product formed when calcium oxide (CaO) is reacted with water (H2O)? 4. What is the chemical formula of the solid formed when carbon dioxide (CO2) is bubbled ...
... What gas is released when carbonates are heated strongly? What solid product is formed when zinc carbonate is heated? What is the name of the product formed when calcium oxide (CaO) is reacted with water (H2O)? 4. What is the chemical formula of the solid formed when carbon dioxide (CO2) is bubbled ...
LECTURE-1 JEO253 PHYSICAL GEOLOGY OVERVIEW
... oceanic lithosphere sinks into a thin upper mantle layer that is well mixed. The cold material is melted, rises, and erupts along mid-ocean ridge spreading centers. • A separate, more sluggish and primitive mantle convective regime is present below 660 km. • The lower ...
... oceanic lithosphere sinks into a thin upper mantle layer that is well mixed. The cold material is melted, rises, and erupts along mid-ocean ridge spreading centers. • A separate, more sluggish and primitive mantle convective regime is present below 660 km. • The lower ...
HONORS EARTH SCIENCE MIDTERM REVIEW
... 3. Explain why the inside of the earth is so hot 4. Compare ocean crust with continental crust 5. Recognize what is happening along convergent, subduction, transform and divergent plate boundaries. Give an example of each. 6. Determine the age of rocks on each side of a rift 7. Describe the theory w ...
... 3. Explain why the inside of the earth is so hot 4. Compare ocean crust with continental crust 5. Recognize what is happening along convergent, subduction, transform and divergent plate boundaries. Give an example of each. 6. Determine the age of rocks on each side of a rift 7. Describe the theory w ...
Needed for Lab 2 Goals of Today’s Lecture Lab 2 • Protractor
... rock is converted to soil minus the change in sediment flux over a landscape element or the sediment flux divergence. ...
... rock is converted to soil minus the change in sediment flux over a landscape element or the sediment flux divergence. ...
The October 13, 2010 Landslides on the Azenge Mountain in
... rains. The landslides were studied using interviews, field observations and laboratory study of the soil samples collected from the major landslide site. Results of the study show that the event that was reported by many local media in Nigeria as volcanic eruption was a spontaneous massive slope mov ...
... rains. The landslides were studied using interviews, field observations and laboratory study of the soil samples collected from the major landslide site. Results of the study show that the event that was reported by many local media in Nigeria as volcanic eruption was a spontaneous massive slope mov ...
File
... For each of the following reactants, use the activity series to determine whether the reaction would take place or not. If no reaction takes. If a reaction does take placeplace, write NR in the blank, write the formulas for the products of the reaction. (Hint: If an active metal replaces the hydroge ...
... For each of the following reactants, use the activity series to determine whether the reaction would take place or not. If no reaction takes. If a reaction does take placeplace, write NR in the blank, write the formulas for the products of the reaction. (Hint: If an active metal replaces the hydroge ...
Synthesis Reactions occur when two of more reactants combine to
... Empirical/Molecular Practice: 1. Zinc form an ionic compound with an oxyanion. The formula is 44.97% Zn and 22.02% S. What is the formula and the name of the compound? 2. Naphthalenedisulfonic acid is found to be 41.7% C, 2.8% H, 22.2% S, and 33.3% O. The molar mass is 288 g/mol. Write the formula. ...
... Empirical/Molecular Practice: 1. Zinc form an ionic compound with an oxyanion. The formula is 44.97% Zn and 22.02% S. What is the formula and the name of the compound? 2. Naphthalenedisulfonic acid is found to be 41.7% C, 2.8% H, 22.2% S, and 33.3% O. The molar mass is 288 g/mol. Write the formula. ...
1. Research agronomists, Beijing Academy of Agriculture and Forest
... was observed among various iron sources. However, there as a significant difference in chlorophyll concentrations among the various iron treatments. MATERIALS AND METHODS A field experiment was conducted in the experiment farm where iron chlorosis in crops was usually observed in the previous years. ...
... was observed among various iron sources. However, there as a significant difference in chlorophyll concentrations among the various iron treatments. MATERIALS AND METHODS A field experiment was conducted in the experiment farm where iron chlorosis in crops was usually observed in the previous years. ...