STUDY MATERIAL 2016-17 CHEMISTRY CLASS XII
... 28. Vacancy defect lowers the density of crystal lattice. 29. Interstitial defect increases the density of crystal. 30. Point defects in the ionic crystal may be classified as: a. Stoichiometric defect (Ratio of cation and anion is same). b. Non Stoichiometric defect (Ratio of cation and anion gets ...
... 28. Vacancy defect lowers the density of crystal lattice. 29. Interstitial defect increases the density of crystal. 30. Point defects in the ionic crystal may be classified as: a. Stoichiometric defect (Ratio of cation and anion is same). b. Non Stoichiometric defect (Ratio of cation and anion gets ...
Mole-Volume Conversion Assignment
... Yesterday’s calculations we found out that when we use 50mL of 5% acetic acid solutions, we require 3.5g of sodium bicarbonate to completely react. Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left ...
... Yesterday’s calculations we found out that when we use 50mL of 5% acetic acid solutions, we require 3.5g of sodium bicarbonate to completely react. Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left ...
Acid-Base Biochemistry
... Under the Brønsted-Lowry definition, both acids and bases are related to the concentration of hydrogen ions present. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions (by accepting them). The acidity or basicity of something therefore can be m ...
... Under the Brønsted-Lowry definition, both acids and bases are related to the concentration of hydrogen ions present. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions (by accepting them). The acidity or basicity of something therefore can be m ...
mod-5-revision-guide-4-transition-metals
... Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solution. Fe2+ is a less strong reducing agent and will only reduce the dichromate to Cr3+ . The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative red ...
... Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solution. Fe2+ is a less strong reducing agent and will only reduce the dichromate to Cr3+ . The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative red ...
HSC Chemistry Syllabus Notes 2007
... These syllabus notes were written to help students to understand and succeed in Chemistry at High School. They are based on the NSW Board of Studies HSC Chemistry Syllabus (Revised 2003). These notes are not a text book and as such I have tried to only include the information that is needed as the s ...
... These syllabus notes were written to help students to understand and succeed in Chemistry at High School. They are based on the NSW Board of Studies HSC Chemistry Syllabus (Revised 2003). These notes are not a text book and as such I have tried to only include the information that is needed as the s ...
Exam 2 Key
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
Unit 4 - Chemical Equilibrium
... _ _ _ _ _ _ _ reaction gets gradually faster. It follows that there must come a time when the reactions are happening in both _ _ _ _ _ _ _ _ _ _ at the same _ _ _ _ and the _ _ _ _ _ _ _ _ _ _ of reactants and products stop changing. This balanced situation is called _ _ _ _ _ _ _ equilibrium. It c ...
... _ _ _ _ _ _ _ reaction gets gradually faster. It follows that there must come a time when the reactions are happening in both _ _ _ _ _ _ _ _ _ _ at the same _ _ _ _ and the _ _ _ _ _ _ _ _ _ _ of reactants and products stop changing. This balanced situation is called _ _ _ _ _ _ _ equilibrium. It c ...
Exam Review
... 8. In the free, or uncombined, state the number of protons in the nucleus of an element must equal the __. a) mass number c) mass number - atomic number b) number of neutrons in the nucleus d) number of electrons present ...
... 8. In the free, or uncombined, state the number of protons in the nucleus of an element must equal the __. a) mass number c) mass number - atomic number b) number of neutrons in the nucleus d) number of electrons present ...
Exam 1
... B. The percentages of ethanol in the vapours increase in order at positions X, Y and Z. C. The percentages of ethanol in the vapours at Y and Z are equal but greater than at X. D. The percentages of ethanol in the vapours at X, Y and Z are equal but greater than 50%. Question 20 The separation and i ...
... B. The percentages of ethanol in the vapours increase in order at positions X, Y and Z. C. The percentages of ethanol in the vapours at Y and Z are equal but greater than at X. D. The percentages of ethanol in the vapours at X, Y and Z are equal but greater than 50%. Question 20 The separation and i ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... This procedure is known as anodic oxidation with H2O2 electrogeneration. The electro-Fenton process involves the enhancement of the oxidizing power of the above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from ...
... This procedure is known as anodic oxidation with H2O2 electrogeneration. The electro-Fenton process involves the enhancement of the oxidizing power of the above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from ...
CHAPTER-7
... Ans. a) H2O --- OHb) NH+4 --- NH3 c) H2CO3 --- HCO-3 d) HS - --- S2e) HCl --- Cl36. OH is a Lewis base. Why? Ans. Since it can donate a pair of electrons. 37. What is the value of ionic product of water at 298K? Ans. 10-14(mol/dm3)2. 38. What is the PH of 10-2 M HCl solution? Ans. PH = 2. 39. The pK ...
... Ans. a) H2O --- OHb) NH+4 --- NH3 c) H2CO3 --- HCO-3 d) HS - --- S2e) HCl --- Cl36. OH is a Lewis base. Why? Ans. Since it can donate a pair of electrons. 37. What is the value of ionic product of water at 298K? Ans. 10-14(mol/dm3)2. 38. What is the PH of 10-2 M HCl solution? Ans. PH = 2. 39. The pK ...
RedOx notes:
... Continue with elements picking their preferred charges (work from outside columns to the inner “valley of confusion”) until there is only one left; if the element is last to choose it must have the charge that makes everything else sum to zero. If you don’t choose first you might not get your first ...
... Continue with elements picking their preferred charges (work from outside columns to the inner “valley of confusion”) until there is only one left; if the element is last to choose it must have the charge that makes everything else sum to zero. If you don’t choose first you might not get your first ...
Organic Acids and Bases and Some of Their Derivatives
... found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group. A carboxylic acid1 is an organic compound that has a carboxyl group2. The carboxyl group is a functional group that contains a carbon–oxygen double bond and an OH grou ...
... found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group. A carboxylic acid1 is an organic compound that has a carboxyl group2. The carboxyl group is a functional group that contains a carbon–oxygen double bond and an OH grou ...
Chemistry II - Mr. Dougan`s Wonderful World of Chemistry
... make about any of the horizontal or vertical rows. For example: In the sodium nitrate row, a typical observation might be this statement: “All nitrates are soluble.” 2. Define the term “solubility product.” Give an example, using one of the precipitates from the data table and the concentrations. Sh ...
... make about any of the horizontal or vertical rows. For example: In the sodium nitrate row, a typical observation might be this statement: “All nitrates are soluble.” 2. Define the term “solubility product.” Give an example, using one of the precipitates from the data table and the concentrations. Sh ...
4Chemical Quantities and Aqueous Reactions
... Without greenhouse gases in the atmosphere, more heat energy would escape, and Earth’s average temperature would be about 60 °F colder than it is now. The temperature outside of my office today would be below 0 °F, and even the sunniest U.S. cities would most likely be covered with snow. However, if ...
... Without greenhouse gases in the atmosphere, more heat energy would escape, and Earth’s average temperature would be about 60 °F colder than it is now. The temperature outside of my office today would be below 0 °F, and even the sunniest U.S. cities would most likely be covered with snow. However, if ...
CHAPTER 16 ACID-BASE EQUILIBRIA AND SOLUBILITY
... Step 2: The molar solubility is the amount of SrF2 that dissolves. From the stoichiometry of the equilibrium equation, you should find that ...
... Step 2: The molar solubility is the amount of SrF2 that dissolves. From the stoichiometry of the equilibrium equation, you should find that ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
chapter twenty-one transition metals and coordination chemistry
... Ligands act as Lewis bases (electron pair donors). f. ...
... Ligands act as Lewis bases (electron pair donors). f. ...
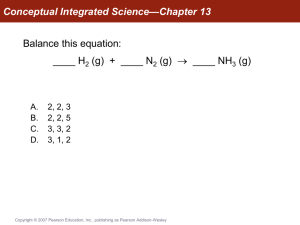
Conceptual Integrated Science—Chapter 13
... What is the relationship between a hydroxide ion and a water molecule? A. A hydroxide ion is a water molecule plus a proton. B. A hydroxide ion and a water molecule are the same things. C. A hydroxide ion is a water molecule minus a hydrogen nucleus. D. A hydroxide ion is a water molecule plus two e ...
... What is the relationship between a hydroxide ion and a water molecule? A. A hydroxide ion is a water molecule plus a proton. B. A hydroxide ion and a water molecule are the same things. C. A hydroxide ion is a water molecule minus a hydrogen nucleus. D. A hydroxide ion is a water molecule plus two e ...
Grossmont College Chemistry 141 Laboratory Manual 6th Edition
... points, but they will differ slightly due to random or indeterminate errors. If you average the data points, this random error is represented by the deviation of each individual measurements from the mean. These are not errors; they are mistakes and cannot be dealt with in any fashion, except perhap ...
... points, but they will differ slightly due to random or indeterminate errors. If you average the data points, this random error is represented by the deviation of each individual measurements from the mean. These are not errors; they are mistakes and cannot be dealt with in any fashion, except perhap ...
5. Coenzyme HAD+ is derived
... -classify organic compounds structurally carbon skeleton and nature of functional groups; -use the rules of nomenclature of chemical compounds; - bind the biological functions of organic molecules with their structure and reactivity; -qualitatively detect unsaturated compounds, amino acids, monosacc ...
... -classify organic compounds structurally carbon skeleton and nature of functional groups; -use the rules of nomenclature of chemical compounds; - bind the biological functions of organic molecules with their structure and reactivity; -qualitatively detect unsaturated compounds, amino acids, monosacc ...
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.The corresponding electrically neutral compound •HO is the hydroxyl radical. The corresponding covalently-bound group -OH of atoms is the hydroxyl group.Hydroxide ion and hydroxyl group are nucleophiles and can act as a catalyst in organic chemistry.Many inorganic substances which bear the word ""hydroxide"" in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxyl groups.