Chemistry Review
... Be able to draw Lewis Structure for molecules and determine bond & molecule polarity. ...
... Be able to draw Lewis Structure for molecules and determine bond & molecule polarity. ...
Writing Lewis Structures for Exceptions to the
... Relatively few molecules formed from two nonmetals contain odd numbers of electrons (e.g. NO, NO2). Since the LE model is based on pairs of electrons, it does not handle oddelectron cases in a natural way. To treat odd-electron molecules, a more sophisticated model are needed – one called the Molecu ...
... Relatively few molecules formed from two nonmetals contain odd numbers of electrons (e.g. NO, NO2). Since the LE model is based on pairs of electrons, it does not handle oddelectron cases in a natural way. To treat odd-electron molecules, a more sophisticated model are needed – one called the Molecu ...
Write this into your supplemental packet opposite page
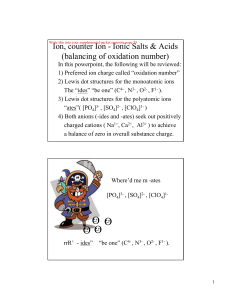
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
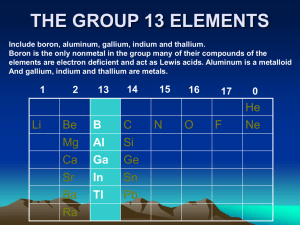
Topic 13 – The periodic table: the transition metals (AHL)
... 7. D; both phosphane (PH3) and water (H2O) contain at least one lone pair of electron on the central atom, enabling them to act as a ligand; the nitrite ion (NO2-) can form coordination complexes in a number of ways; 8. D; the color of transition metal ions is associated with partially filled d or ...
... 7. D; both phosphane (PH3) and water (H2O) contain at least one lone pair of electron on the central atom, enabling them to act as a ligand; the nitrite ion (NO2-) can form coordination complexes in a number of ways; 8. D; the color of transition metal ions is associated with partially filled d or ...
d-block chemistry – general considerations
... Coordination sphere acts as a unit. Ions outside brackets balance charge – free ions in solution Metal can have from one up to 16 ligands attached to it 4 and 6 most common Additional water molecules may be added to coordination sphere when compounds are dissolved in water. Coordination Number of Me ...
... Coordination sphere acts as a unit. Ions outside brackets balance charge – free ions in solution Metal can have from one up to 16 ligands attached to it 4 and 6 most common Additional water molecules may be added to coordination sphere when compounds are dissolved in water. Coordination Number of Me ...
The structure of Matter
... O The electron may be more strongly attracted to one of the atoms and spend more time revolving around that nucleus instead of the other. O This results in a positive and negative end for the compound (the end with the electron most of the time is negative…). O This is called a polar bond. ...
... O The electron may be more strongly attracted to one of the atoms and spend more time revolving around that nucleus instead of the other. O This results in a positive and negative end for the compound (the end with the electron most of the time is negative…). O This is called a polar bond. ...
6th generation single crystal superalloy TMS-238
... The oxidation property was investigated and is shown in Fig. 3. TMS-196 showed an improvement over TMS-138A and has excellent oxidation resistance compared with other 4th and 5th generation superalloys [11]: however, it shows a large decrease in mass in the cyclic oxidation test because of scale spa ...
... The oxidation property was investigated and is shown in Fig. 3. TMS-196 showed an improvement over TMS-138A and has excellent oxidation resistance compared with other 4th and 5th generation superalloys [11]: however, it shows a large decrease in mass in the cyclic oxidation test because of scale spa ...