Atoms, Molecules, and Ions Chemistry Timeline #1
... Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that a ...
... Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that a ...
Atomic Structure -
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
Practice Packet
... *You can round the masses given to you or use them as given – just be consistent! 1) Element X exists in three isotopic forms. The isotopic mixture consists of 10.0% 10X, 20.0% 11X, and 70.0% 12X. What is the average atomic mass of this element? ...
... *You can round the masses given to you or use them as given – just be consistent! 1) Element X exists in three isotopic forms. The isotopic mixture consists of 10.0% 10X, 20.0% 11X, and 70.0% 12X. What is the average atomic mass of this element? ...
Atoms, Molecules, and Ions The Evolution of the Atomic Model (from
... chemical systems. These became known as Dalton’s Atomic Theory and he proposed this the year 1803. The primary difference between Dalton’s theory and previous ones was that Dalton’s was based on reproducible laboratory evidence. ...
... chemical systems. These became known as Dalton’s Atomic Theory and he proposed this the year 1803. The primary difference between Dalton’s theory and previous ones was that Dalton’s was based on reproducible laboratory evidence. ...
atomic mass - Cloudfront.net
... Atomic Number • Atomic Number – The number of protons in an atom’s nucleus. It is used to determine and #1=H– describe each #2 = He – element #3 = Li – ...
... Atomic Number • Atomic Number – The number of protons in an atom’s nucleus. It is used to determine and #1=H– describe each #2 = He – element #3 = Li – ...
What is an atom?
... abundant form of hydrogen does not have a neutron. However, in rare instances isotopes form. Below are the isotopes of ...
... abundant form of hydrogen does not have a neutron. However, in rare instances isotopes form. Below are the isotopes of ...
3lou3atch - Teach-n-Learn-Chem
... energy levels = the possible electron orbits of an atom ground state = exists when an atom is energetically stable excited state = exists when electrons absorb energy, are moved to higher levels, and the atom become energetically unstable How do these definitions describe an electron in an atom in t ...
... energy levels = the possible electron orbits of an atom ground state = exists when an atom is energetically stable excited state = exists when electrons absorb energy, are moved to higher levels, and the atom become energetically unstable How do these definitions describe an electron in an atom in t ...
Notes powerpoint
... • For example, four out of five atoms of boron are boron-11, and one out of five is boron-10. • To find the weighted-average or the average atomic mass of boron, you would solve the following equation: ...
... • For example, four out of five atoms of boron are boron-11, and one out of five is boron-10. • To find the weighted-average or the average atomic mass of boron, you would solve the following equation: ...
Ch-03 Notes ppt
... identical in size, mass and other properties different from those of the other elements 3) Atoms cannot be subdivided, created or destroyed 4) Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative number and types of atoms 5) I ...
... identical in size, mass and other properties different from those of the other elements 3) Atoms cannot be subdivided, created or destroyed 4) Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative number and types of atoms 5) I ...
1.2--NOTES--Basic Atomic Structure
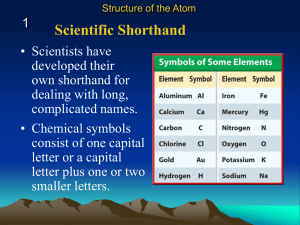
... • all symbols must either be one capital letter, or one capital and one lowercase. •atomic number = number of p+. Written in bottomleft-hand corner of symbol. Identifies the element. •mass number = number of p+ & n0. Identifies the isotope. Written in top-left-hand corner. •average atomic mass = wei ...
... • all symbols must either be one capital letter, or one capital and one lowercase. •atomic number = number of p+. Written in bottomleft-hand corner of symbol. Identifies the element. •mass number = number of p+ & n0. Identifies the isotope. Written in top-left-hand corner. •average atomic mass = wei ...
Answer key
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
File
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
... Protons and neutrons are found in the center of the atom, called the nucleus. The electrons move about in the electron cloud that surrounds the nucleus. 46. Which subatomic particle(s) defines the identity of the atom? Protons 47. Which subatomic particle(s) determines chemical properties? electrons ...
V. Chemical reactions
... d. What particles are in equal numbers in a neutral atom? protons & electrons e. How is the number of protons determined? by atomic number f. How is the number of neutrons determined? subtract atomic number from mass number g. How is the number of electrons determined in a neutral atom? Equal to the ...
... d. What particles are in equal numbers in a neutral atom? protons & electrons e. How is the number of protons determined? by atomic number f. How is the number of neutrons determined? subtract atomic number from mass number g. How is the number of electrons determined in a neutral atom? Equal to the ...
number of protons - Waukee Community School District Blogs
... Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. ...
... Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. ...
Atoms: The Building Blocks of Matter
... shows that 2 molecules (made of 4 atoms) of hydrogen and 1 molecule (made of 2 atoms) of oxygen produce 2 molecules of water. The total mass of the product, water, is equal to the sum of the masses of each of the reactants, hydrogen and oxygen. What parts of Dalton’s atomic theory are illustrated by ...
... shows that 2 molecules (made of 4 atoms) of hydrogen and 1 molecule (made of 2 atoms) of oxygen produce 2 molecules of water. The total mass of the product, water, is equal to the sum of the masses of each of the reactants, hydrogen and oxygen. What parts of Dalton’s atomic theory are illustrated by ...
Study Guide: Elements, Compounds, Mixtures Physical Properties
... Physical Properties: Can be observed or measured without chemically changing a substance. Can be used to identify an unknown substance (some are more useful for this purpose than others, such as: specific heat, density (mass/volume), melting point, boiling point) Are: malleability, solubility, densi ...
... Physical Properties: Can be observed or measured without chemically changing a substance. Can be used to identify an unknown substance (some are more useful for this purpose than others, such as: specific heat, density (mass/volume), melting point, boiling point) Are: malleability, solubility, densi ...
ATOMS: THE BUILDING BLOCKS OF MATTER
... Atoms of different elements combine in In chemical reactions, atoms are LAW OF MULTIPLE PROPORTION Atoms of different elements combine in simple whole number ratios to form chemical compounds Not all aspects of Dalton’s atomic theory have proven to be correct Dalton’s atomic theory have been modifie ...
... Atoms of different elements combine in In chemical reactions, atoms are LAW OF MULTIPLE PROPORTION Atoms of different elements combine in simple whole number ratios to form chemical compounds Not all aspects of Dalton’s atomic theory have proven to be correct Dalton’s atomic theory have been modifie ...
Atomic Structure Notes
... arranged in order of increasing atomic masses, certain properties would repeat every eighth element. He arranged the elements into seven groups of seven elements and called his system the "law of octaves.". In the 1860’s a German Chemist named Lothar Meyer was developing a periodic table based on th ...
... arranged in order of increasing atomic masses, certain properties would repeat every eighth element. He arranged the elements into seven groups of seven elements and called his system the "law of octaves.". In the 1860’s a German Chemist named Lothar Meyer was developing a periodic table based on th ...
Element - Faculty
... Chemical formulas can be determined by measuring the mass of each element present in a sample of the compound. The mass of each element (grams) is converted to number of moles, or molecules of each element presenting the compound. You will need to do such calculations in order to determine the amoun ...
... Chemical formulas can be determined by measuring the mass of each element present in a sample of the compound. The mass of each element (grams) is converted to number of moles, or molecules of each element presenting the compound. You will need to do such calculations in order to determine the amoun ...
Chapter 4: Chemical Reactions Elements can be characterized as
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
Teaching/Chemistry/Chemistry Lesson Plans 04
... of groups, periods, and the transition metals in the periodic table o In the mid-1800’s 70 elements had been discovered o Russian Dmitri Mendeleev was the first person to arrange the elements in columns so that elements with similar properties were side by side For missing elements, blanks were left ...
... of groups, periods, and the transition metals in the periodic table o In the mid-1800’s 70 elements had been discovered o Russian Dmitri Mendeleev was the first person to arrange the elements in columns so that elements with similar properties were side by side For missing elements, blanks were left ...
atoms - schultz915
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Chapter 3 - Industrial ISD
... 4. Which scientist discovered the electrons? 5. Which scientist discovered the nucleus? 6. Which scientist discovered that electrons where in energy levels? 7. Which scientist said atoms where neutral because they had equal number of protons and electrons? ...
... 4. Which scientist discovered the electrons? 5. Which scientist discovered the nucleus? 6. Which scientist discovered that electrons where in energy levels? 7. Which scientist said atoms where neutral because they had equal number of protons and electrons? ...