Pure Substances and Mixtures
... – Atoms are the basic building blocks of all matter (the smallest particle of matter) – There are different kinds of atoms. ...
... – Atoms are the basic building blocks of all matter (the smallest particle of matter) – There are different kinds of atoms. ...
Ch. 2 Chemical Basis of the Body (pp. 26-33)
... Radioisotopes *By losing subatomic particles, energy is released called radioactive decay. *This energy can penetrate and destroy tissues *Since rapidly dividing cells are more sensitive to destructive effects of radiation, this is why radiation is good on cancer. ...
... Radioisotopes *By losing subatomic particles, energy is released called radioactive decay. *This energy can penetrate and destroy tissues *Since rapidly dividing cells are more sensitive to destructive effects of radiation, this is why radiation is good on cancer. ...
Notes matter energy
... Pure substances – made up of one substance that cannot be physically separated. 2 types are: Compounds – can be chemically separated into elements Elements – cannot be broken down by chemical reactions. Known elements appear on the periodic table of the elements. Elements on the periodic table have ...
... Pure substances – made up of one substance that cannot be physically separated. 2 types are: Compounds – can be chemically separated into elements Elements – cannot be broken down by chemical reactions. Known elements appear on the periodic table of the elements. Elements on the periodic table have ...
Chapter 5 The Structure of the Atom
... 1. The negative charges came from within the atom. 2. A particle of matter smaller than the atom had to exist. 3. The atom was divisible. 4. Called the negatively particles “corpuscles” (now called electrons) 5. Since the gas was known to be neutral, there had to be positive charged particles in the ...
... 1. The negative charges came from within the atom. 2. A particle of matter smaller than the atom had to exist. 3. The atom was divisible. 4. Called the negatively particles “corpuscles” (now called electrons) 5. Since the gas was known to be neutral, there had to be positive charged particles in the ...
Linking Asteroids and Meteorites through Reflectance Spectroscopy
... 26/5925968-see-the-worlds-smallest-periodictable ...
... 26/5925968-see-the-worlds-smallest-periodictable ...
elements in a family have the same number of
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Chapter 3 PowerPoint
... thought all matter was continuous. Democritus does not get credit for discovering the atom because he had no scientific evidence to back it up. ...
... thought all matter was continuous. Democritus does not get credit for discovering the atom because he had no scientific evidence to back it up. ...
Atomictheory
... • Scientists determined that electrons do not orbit the the nucleus like a planet. • Electrons can be found anywhere in a cloud like region around the nucleus. • An electrons movement is related to its energy level- specific amount of energy it ...
... • Scientists determined that electrons do not orbit the the nucleus like a planet. • Electrons can be found anywhere in a cloud like region around the nucleus. • An electrons movement is related to its energy level- specific amount of energy it ...
Semester 1 Final Exam Study Guide
... 29. Fill in the chart below for the following neutral atoms. Element ...
... 29. Fill in the chart below for the following neutral atoms. Element ...
So where did all the matter on Earth come from - Bennatti
... oxygen atoms and you still have hydrogen and oxygen atoms after the chemical reaction has occurred…they have simply bonded together to make a water molecule. Nuclear reactions like those that occur in a nuclear power plant or in a star are very different from chemical reactions. They are called nucl ...
... oxygen atoms and you still have hydrogen and oxygen atoms after the chemical reaction has occurred…they have simply bonded together to make a water molecule. Nuclear reactions like those that occur in a nuclear power plant or in a star are very different from chemical reactions. They are called nucl ...
Review: theory vs law the atomic theory contributions of early scientists
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
Atomic Structure - Teach-n-Learn-Chem
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
Unit 2 Atomic Structure
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
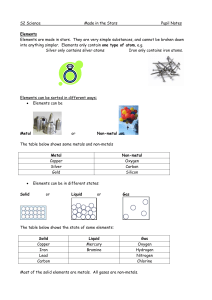
Made in the Stars Notes
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
File
... H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matter is made up of small particles called atoms 2. Atoms can’t be created or destroyed. 3. Atoms of the same element have the same size and mass, but are differe ...
... H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matter is made up of small particles called atoms 2. Atoms can’t be created or destroyed. 3. Atoms of the same element have the same size and mass, but are differe ...
Packet
... 39. For the following elements, provide information as to group, block and metal status as indicated on the top of the chart. Element ...
... 39. For the following elements, provide information as to group, block and metal status as indicated on the top of the chart. Element ...
Unit 4 Study Guide Groups to know: Alkali Metals
... Modified Theory: The atom has a very dense, positively charged core (the nucleus) Niels Bohr - Bohr Model -proposed that electrons orbit the nucleus in distinct energy levels like planets around the sun Modern Atomic Theory -Electrons move randomly around the nucleus in the electron cloud -We cannot ...
... Modified Theory: The atom has a very dense, positively charged core (the nucleus) Niels Bohr - Bohr Model -proposed that electrons orbit the nucleus in distinct energy levels like planets around the sun Modern Atomic Theory -Electrons move randomly around the nucleus in the electron cloud -We cannot ...
Chemistry Study Guide What is matter made of? Matter is anything
... properties that are the same or very similar. The elements in each group also have the same number of electrons in their outer shell. The horizontal rows are called periods. The elements in each period are arranged by atomic number and have the same number of electron shells around the nucleus. Eac ...
... properties that are the same or very similar. The elements in each group also have the same number of electrons in their outer shell. The horizontal rows are called periods. The elements in each period are arranged by atomic number and have the same number of electron shells around the nucleus. Eac ...
Linking Asteroids and Meteorites through Reflectance
... Periodic Table • A chart in which all known elements are listed in order of atomic number • http://theodoregray.com/periodictable/ ...
... Periodic Table • A chart in which all known elements are listed in order of atomic number • http://theodoregray.com/periodictable/ ...
Chemistry Unit Test Review
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
Chapter 5
... Charges are of two types: Positive and Negative Unlike charges attract and like charges repel Charges may be transferred by contact or induction The closer two object are, the greater the force of attraction ...
... Charges are of two types: Positive and Negative Unlike charges attract and like charges repel Charges may be transferred by contact or induction The closer two object are, the greater the force of attraction ...
Topic 3 Review
... • He also found the charge to mass ratio of the electrons. • Millikan measured the charge of the electrons. • The mass of an electron was found to be about 1/2000! the mass of a hydrogen atom. ...
... • He also found the charge to mass ratio of the electrons. • Millikan measured the charge of the electrons. • The mass of an electron was found to be about 1/2000! the mass of a hydrogen atom. ...