Chemistry Notes (pg. # 1)
... Question: How did scientists determine that the atom is made up of ____________, ____________, and ____________? - Also, how did they determine that there is a ____________ and an electron “__________”? Evidence for the Negative Electron: Cathode Ray T_____ Experiment: The Cathode Ray T______ experi ...
... Question: How did scientists determine that the atom is made up of ____________, ____________, and ____________? - Also, how did they determine that there is a ____________ and an electron “__________”? Evidence for the Negative Electron: Cathode Ray T_____ Experiment: The Cathode Ray T______ experi ...
Answer = 1.81 x 10 24 molecules
... • They expected the particles to go through but some of them deflected back • Later they found that this was because atoms have a very small, very dense area with a positive charge called the nucleus ...
... • They expected the particles to go through but some of them deflected back • Later they found that this was because atoms have a very small, very dense area with a positive charge called the nucleus ...
Atomic Theory Powerpoint
... Discovery of Nucleus Characteristics of “Powerful Force”: 1. dense- since it was strong enough to deflect particle 2. small- only 1/8000 hit the force dead on and bounced back 3. positively charged- since there was a repulsion between force and alpha particles ...
... Discovery of Nucleus Characteristics of “Powerful Force”: 1. dense- since it was strong enough to deflect particle 2. small- only 1/8000 hit the force dead on and bounced back 3. positively charged- since there was a repulsion between force and alpha particles ...
The Atom - Exam #2 Review
... d. Bohr Planetary Model e. Chadwick (has neutrons in nucleus) f. Modern (Schrödinger and Heisenberg) Quantum Mechanical Model ...
... d. Bohr Planetary Model e. Chadwick (has neutrons in nucleus) f. Modern (Schrödinger and Heisenberg) Quantum Mechanical Model ...
Chemistry Comes Alive: Part A
... • Atomic number = number of protons in nucleus Identifying Elements • Mass number = mass of the protons and neutrons • Mass numbers of atoms of an element are not all identical • Isotopes are structural variations of elements that differ in the number of neutrons they contain ...
... • Atomic number = number of protons in nucleus Identifying Elements • Mass number = mass of the protons and neutrons • Mass numbers of atoms of an element are not all identical • Isotopes are structural variations of elements that differ in the number of neutrons they contain ...
Test Review with answer key and explanations
... The atomic number on the periodic table equals the number of protons. So an atom with 20 protons has an atomic number of 20. The element with the atomic number of 20 on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the ch ...
... The atomic number on the periodic table equals the number of protons. So an atom with 20 protons has an atomic number of 20. The element with the atomic number of 20 on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the ch ...
Chapters 1-4 Numbers and Measurements in Chemistry Units SI
... • Heaviest atom is ~260 amu (4x10-22 g) • Largest atom is 500 pm across. • Typical C-C bond length 154 pm ...
... • Heaviest atom is ~260 amu (4x10-22 g) • Largest atom is 500 pm across. • Typical C-C bond length 154 pm ...
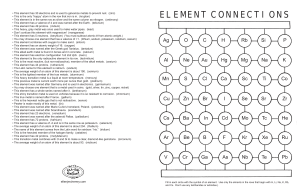
element connections
... • This element has 5 neutrons. (beryllium) (You must subtract atomic # from atomic weight.) • You may choose one element that has a valence of +1. (lithium, sodium, potassium, rubidium, cesium) • This element combines with oxygen to make sand. (silicon) • This element has an atomic weight of 16. (ox ...
... • This element has 5 neutrons. (beryllium) (You must subtract atomic # from atomic weight.) • You may choose one element that has a valence of +1. (lithium, sodium, potassium, rubidium, cesium) • This element combines with oxygen to make sand. (silicon) • This element has an atomic weight of 16. (ox ...
Blank Quiz - Fort Bend ISD
... The atomic number on the periodic table equals the number of protons. So an atom with 20 protons has an atomic number of 20. The element with the atomic number of 20 on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the ch ...
... The atomic number on the periodic table equals the number of protons. So an atom with 20 protons has an atomic number of 20. The element with the atomic number of 20 on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the ch ...
q2-w4-hw-atomic-vocab - PARADE 7/8 STEM
... 15. The _______________ is always a whole number. A. Atomic number B. Mass number C. Atomic mass 16. To get the number of neutrons for an element, we take the _____ and subtract the ______. A. mass number minus the atomic number B. atomic number minus the mass number 17. In the case of Sodium, calcu ...
... 15. The _______________ is always a whole number. A. Atomic number B. Mass number C. Atomic mass 16. To get the number of neutrons for an element, we take the _____ and subtract the ______. A. mass number minus the atomic number B. atomic number minus the mass number 17. In the case of Sodium, calcu ...
File
... - Lothar Meyer of Germany independently presented a similar table in 1870. - Henry Moseley, a British physicist, carried on the work of Mendeleev after his death and arranged the elements according to atomic no. instead of atomic mass. - Indicated in the present day table are the element symbol, ato ...
... - Lothar Meyer of Germany independently presented a similar table in 1870. - Henry Moseley, a British physicist, carried on the work of Mendeleev after his death and arranged the elements according to atomic no. instead of atomic mass. - Indicated in the present day table are the element symbol, ato ...
Bonding
... ✦ Metals react with nonmetals ✦ Ions paired have lower energy (greater stability) than separated ions Covalent ✦ Electrons are shared by nuclei ✦ Pure covalent (nonpolar covalent) - electrons are shared evenly ✦ Polar covalent - electrons shared unequally ...
... ✦ Metals react with nonmetals ✦ Ions paired have lower energy (greater stability) than separated ions Covalent ✦ Electrons are shared by nuclei ✦ Pure covalent (nonpolar covalent) - electrons are shared evenly ✦ Polar covalent - electrons shared unequally ...
iClicker Participation Question
... we move from left right on the periodic table, shielding of the nucleus by inner (core) electrons remains constant. (Shielding by other outer electrons is minimal.) ...
... we move from left right on the periodic table, shielding of the nucleus by inner (core) electrons remains constant. (Shielding by other outer electrons is minimal.) ...
Document
... 17. compound - A form of matter made by chemically combining two or more different elements. 18. mixture- A combination of two or more substances that are not chemically combined and can be separated by physical means. 19. chemical formula- Notation made up of the symbols of the elements and number ...
... 17. compound - A form of matter made by chemically combining two or more different elements. 18. mixture- A combination of two or more substances that are not chemically combined and can be separated by physical means. 19. chemical formula- Notation made up of the symbols of the elements and number ...
Exam #2 Review
... Atomic Model History – MAKE SURE YOU CAN MATCH EACH SCIENTIST TO HIS MODEL!! 1. Draw and name each scientist’s model of the atom: a. Dalton Billiard Ball Model ...
... Atomic Model History – MAKE SURE YOU CAN MATCH EACH SCIENTIST TO HIS MODEL!! 1. Draw and name each scientist’s model of the atom: a. Dalton Billiard Ball Model ...
Atomic Structure Worksheet
... 1. The extremely low-mass, negatively charged particle found outside the nucleus is called an electron. 2. The number of protons in the nucleus of the atom is indicated by the atomic number. 3. The symbol for an element is either a capital letter or possibly a capital letter followed by a lower case ...
... 1. The extremely low-mass, negatively charged particle found outside the nucleus is called an electron. 2. The number of protons in the nucleus of the atom is indicated by the atomic number. 3. The symbol for an element is either a capital letter or possibly a capital letter followed by a lower case ...
Chapter 5/6 Notes
... BIG SOLUTION: In 1913, Neils Bohr (Danish), stated that electrons could occupy fixed Chem Stud orbitals without giving off energy. ...
... BIG SOLUTION: In 1913, Neils Bohr (Danish), stated that electrons could occupy fixed Chem Stud orbitals without giving off energy. ...
1 Atomic Mass
... An element s atomic mass (listed in Periodic Table) are weighted averages for the naturally occurring mixtures of different isotopes of that element ...
... An element s atomic mass (listed in Periodic Table) are weighted averages for the naturally occurring mixtures of different isotopes of that element ...
Periodic table and the atom
... Results = The atom was a ball of positive charges with small negative particles called electrons **DISCOVERED THE ELECTRON*** Model of the atom = PLUM PUDDING MODEL ...
... Results = The atom was a ball of positive charges with small negative particles called electrons **DISCOVERED THE ELECTRON*** Model of the atom = PLUM PUDDING MODEL ...
Atomic number
... often 3usually 2- always 1Charge = 8 - group number Group VIIIA: no charge -noble gases ...
... often 3usually 2- always 1Charge = 8 - group number Group VIIIA: no charge -noble gases ...
Honors Chemistry Chapter 6 Student Notes
... f block elements are called inner transition elements - they were put into their current position by Glenn Seaborg - the only living person ever to have an element named after himself. ...
... f block elements are called inner transition elements - they were put into their current position by Glenn Seaborg - the only living person ever to have an element named after himself. ...
1020 Chapter 4 Lecture Notes
... Protons and neutrons are held together to form nuclei by the strong nuclear force. Energy must be expended to separate a nucleus into individual nucleons. At the subatomic level, energy and mass are equivalent. When a system gains energy, it gains mass. When a system loses energy, its mass decreases ...
... Protons and neutrons are held together to form nuclei by the strong nuclear force. Energy must be expended to separate a nucleus into individual nucleons. At the subatomic level, energy and mass are equivalent. When a system gains energy, it gains mass. When a system loses energy, its mass decreases ...
Atoms and the Periodic Table PowerPoint
... began working on his great achievement: the periodic table of the elements. By arranging all of the 63 elements then known by their atomic weights, he managed to organize them into groups possessing similar properties. Where a gap existed in the table, he predicted a new element would one day be fou ...
... began working on his great achievement: the periodic table of the elements. By arranging all of the 63 elements then known by their atomic weights, he managed to organize them into groups possessing similar properties. Where a gap existed in the table, he predicted a new element would one day be fou ...
HW Problems
... 6. An atom of rhodium (Rh) has a diameter of 2.7 x 10-8 cm. a. How many Rh atoms would you place next to one another to span a distance of 6 μm? b. If Rh atoms are spheres, what is the volume of a single atom (m3)? 7. Label each of these statements as true or false? a. The nucleus of an atom contain ...
... 6. An atom of rhodium (Rh) has a diameter of 2.7 x 10-8 cm. a. How many Rh atoms would you place next to one another to span a distance of 6 μm? b. If Rh atoms are spheres, what is the volume of a single atom (m3)? 7. Label each of these statements as true or false? a. The nucleus of an atom contain ...