Chapter 2: Atoms, Molecules, and Ions
... 3. ___________________are formed when _____________of different elements unite in _______________________________________ratios. 4. Chemical _______________involve ___________________________________; no _____________are created, destroyed or broken apart in a chemical reaction. ...
... 3. ___________________are formed when _____________of different elements unite in _______________________________________ratios. 4. Chemical _______________involve ___________________________________; no _____________are created, destroyed or broken apart in a chemical reaction. ...
Slide 1
... • Today, we still use the term element, though in a different way. For example, we still believe that most substances are built up from simpler ones. ...
... • Today, we still use the term element, though in a different way. For example, we still believe that most substances are built up from simpler ones. ...
Isotope Practice Worksheet
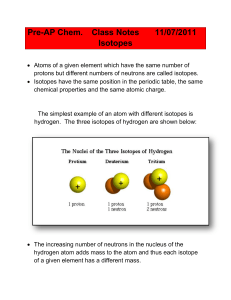
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
Advances in Atomic Theory
... of ___________ but the number of _____________ may vary. Isotopes - Atoms of the ________ element that have different numbers of __________________. Isotopes can be written two ways: 1. The name of the element followed by the mass number. Ex. Carbon - 12 2. The chemical symbol with the mass number a ...
... of ___________ but the number of _____________ may vary. Isotopes - Atoms of the ________ element that have different numbers of __________________. Isotopes can be written two ways: 1. The name of the element followed by the mass number. Ex. Carbon - 12 2. The chemical symbol with the mass number a ...
Atomic Theory - Hicksville Public Schools
... Since atoms cannot be divided or destroyed, then a chemical change is a rearrangement of atoms. a. The total mass of substances in a reaction does not change. C. Law of Definite Proportions (Joseph Proust - 1799) ...
... Since atoms cannot be divided or destroyed, then a chemical change is a rearrangement of atoms. a. The total mass of substances in a reaction does not change. C. Law of Definite Proportions (Joseph Proust - 1799) ...
atomic
... • protons and neutrons are made up of quarks – which three quarks – determines whether it becomes a proton or a neutron. ...
... • protons and neutrons are made up of quarks – which three quarks – determines whether it becomes a proton or a neutron. ...
periodic table
... both reflect strength of forces between their molecules: strongest when valence shells are half empty (middle of periodic table) ...
... both reflect strength of forces between their molecules: strongest when valence shells are half empty (middle of periodic table) ...
Atomic Structure - Learn District 196
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
File - Mr. Meyer`s Science Page
... atomic number, similarities in their properties will emerge in a regular pattern. Objective 2: Explain why some atoms gain or lose electrons to form ions. ...
... atomic number, similarities in their properties will emerge in a regular pattern. Objective 2: Explain why some atoms gain or lose electrons to form ions. ...
Atomic Structure
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
Chapter 2 Atoms, Molecules, and Ions
... voltage produces radiation, which is called a cathode ray, because it comes from the negative electrode, or cathode. • The rays could not be seen, but detected. Depending on which gas is in the tube, the ray will give off a certain colored light. ...
... voltage produces radiation, which is called a cathode ray, because it comes from the negative electrode, or cathode. • The rays could not be seen, but detected. Depending on which gas is in the tube, the ray will give off a certain colored light. ...
Chapter 5
... Beta Decay The emission of an electron from the nucleus and the transformation of the atom into a different element with the next higher atomic # is the result. ...
... Beta Decay The emission of an electron from the nucleus and the transformation of the atom into a different element with the next higher atomic # is the result. ...
Module 3 - Tuskegee University
... atomic number instead of atomic mass, which is the current periodic table. Elements in each group (vertical column) have similar properties because they have the same number of valence electrons for chemical bonding. The number of valence electrons can be found from I, II, … VIII, group number. ...
... atomic number instead of atomic mass, which is the current periodic table. Elements in each group (vertical column) have similar properties because they have the same number of valence electrons for chemical bonding. The number of valence electrons can be found from I, II, … VIII, group number. ...
Powerpoint - Tuskegee University
... atomic number instead of atomic mass, which is the current periodic table. Elements in each group (vertical column) have similar properties because they have the same number of valence electrons for chemical bonding. The number of valence electrons can be found from I, II, … VIII, group number. ...
... atomic number instead of atomic mass, which is the current periodic table. Elements in each group (vertical column) have similar properties because they have the same number of valence electrons for chemical bonding. The number of valence electrons can be found from I, II, … VIII, group number. ...
Regents questions
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
The Modern View of Atomic Structure
... A radioactive substance is placed in a shield containing a small hole so that a beam of radiation is emitted from the hole. The radiation is passed between two electrically charged plates and detected. Three spots are noted on the detector: a spot in the direction of the positive plate, a spot which ...
... A radioactive substance is placed in a shield containing a small hole so that a beam of radiation is emitted from the hole. The radiation is passed between two electrically charged plates and detected. Three spots are noted on the detector: a spot in the direction of the positive plate, a spot which ...
Unit 4 Packet
... 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. 15. How many protons, neutrons, and electrons are in an atom of Ac–221? 16. How many electrons, neutrons, and protons are ...
... 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. 15. How many protons, neutrons, and electrons are in an atom of Ac–221? 16. How many electrons, neutrons, and protons are ...
ATOMS - Mr. Deets
... • Shoots alpha particles (Helium atoms) at gold foil • Expected to pass right through • Particles are deflected • Leads to idea of a dense positively charged center with e- orbiting around it ...
... • Shoots alpha particles (Helium atoms) at gold foil • Expected to pass right through • Particles are deflected • Leads to idea of a dense positively charged center with e- orbiting around it ...
The evolution of Atomic Theory
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
Introduction to the Atom
... The amu is the measurement of weight of an atom. The amu is the atom mass units. The amu system of measurement assigns one (1) amu to both protons and neutrons. The atomic number represents the number of protons an atom contains in the nucleus. An example is oxygen. The atomic number of oxygen is ...
... The amu is the measurement of weight of an atom. The amu is the atom mass units. The amu system of measurement assigns one (1) amu to both protons and neutrons. The atomic number represents the number of protons an atom contains in the nucleus. An example is oxygen. The atomic number of oxygen is ...
Atom notes - WordPress.com
... 2. The neutron was basically equal in mass to the proton but had _______ ____________________ charge. NEILS BOHR (1914) 1. Concluded that ________________ moved around the nucleus in definite orbits or ___________________________. DALTON REVISITED 1. Atom was indivisible. _____________ 2. All elemen ...
... 2. The neutron was basically equal in mass to the proton but had _______ ____________________ charge. NEILS BOHR (1914) 1. Concluded that ________________ moved around the nucleus in definite orbits or ___________________________. DALTON REVISITED 1. Atom was indivisible. _____________ 2. All elemen ...