Electron Proton Neutron
... The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11. ...
... The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11. ...
1) Molecular Compounds
... Thomson could not find a positively charged particle, so he believed that the electrons were like plums embedded in a positively charged “pudding,” thus it became known as the “plum pudding” model. 2) Robert Millikan added to our understanding of the electron with his oil drop apparatus. Millikan s ...
... Thomson could not find a positively charged particle, so he believed that the electrons were like plums embedded in a positively charged “pudding,” thus it became known as the “plum pudding” model. 2) Robert Millikan added to our understanding of the electron with his oil drop apparatus. Millikan s ...
Document
... Carbon, with an atomic number of 6, has three isotopes; one with a mass number of 12, one with a mass number of 13 and one with a mass number of ...
... Carbon, with an atomic number of 6, has three isotopes; one with a mass number of 12, one with a mass number of 13 and one with a mass number of ...
Atomic
... • In an ion (with a positive or negative charge), the number of electrons is different from the number of protons. To find the number of electrons, subtract the charge from the number of protons the atom has • # protons – charge = # electrons ...
... • In an ion (with a positive or negative charge), the number of electrons is different from the number of protons. To find the number of electrons, subtract the charge from the number of protons the atom has • # protons – charge = # electrons ...
04 Atom notes
... The atomic number defines the element! The symbol for the atomic number is Z. Mass number ________________________________________________________ The mass number defines the isotope of that element. The symbol for mass number is A. Nuclear Symbol: ...
... The atomic number defines the element! The symbol for the atomic number is Z. Mass number ________________________________________________________ The mass number defines the isotope of that element. The symbol for mass number is A. Nuclear Symbol: ...
Chapter 16 - Structure of an Atom - from class 4/13/15
... • Homework for the week is on the board. • You will need your binder open and ready to take notes today – there will also be a worksheet to take notes on as well • Blue students will be joining us so it is important to not talk, stay focused and get the information you need today. Oh and squeeze in ...
... • Homework for the week is on the board. • You will need your binder open and ready to take notes today – there will also be a worksheet to take notes on as well • Blue students will be joining us so it is important to not talk, stay focused and get the information you need today. Oh and squeeze in ...
Notes 4.3 filled in
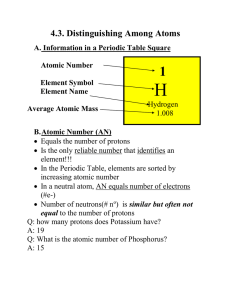
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
Atoms, Molecules and Ions
... 1. Elements are composed of extremely small particles called atoms. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. 2. Compounds are composed of atoms of more than one element ...
... 1. Elements are composed of extremely small particles called atoms. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. 2. Compounds are composed of atoms of more than one element ...
The Material World: An Introduction to Chemistry 1. Modern Model of
... explain why even the thin lines in an emission spectrum could be resolved into more fine lines, and they had to include the discovery of neutrons into their model. The atom is the smallest unit of an element that still behaves like the entire element, but that's not to say that the smaller parts do ...
... explain why even the thin lines in an emission spectrum could be resolved into more fine lines, and they had to include the discovery of neutrons into their model. The atom is the smallest unit of an element that still behaves like the entire element, but that's not to say that the smaller parts do ...
Review for Test
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
Subatomic Particles
... The ancient Greek philosopher Democritus coined what word for a tiny piece of matter that cannot be divided? a) Element b) Electron c) Atom d) Molecule Dalton’s theory (~1800; based on behavior of gasses) included all but one of the following points. Which is not from Dalton? a) All elements are com ...
... The ancient Greek philosopher Democritus coined what word for a tiny piece of matter that cannot be divided? a) Element b) Electron c) Atom d) Molecule Dalton’s theory (~1800; based on behavior of gasses) included all but one of the following points. Which is not from Dalton? a) All elements are com ...
Unit 3 Notes, Practice, and Review
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
Atomic Theory 1
... The complete atomic symbol’s mass number’ (A) and the respective Element’s ‘box weight’ in the periodic table do NOT convey the same information. The complete atomic symbol denotes the mass of ONE isotope of the element in amu, while the p. table gives is the average mass of ALL isotopes of the elem ...
... The complete atomic symbol’s mass number’ (A) and the respective Element’s ‘box weight’ in the periodic table do NOT convey the same information. The complete atomic symbol denotes the mass of ONE isotope of the element in amu, while the p. table gives is the average mass of ALL isotopes of the elem ...
The Development of Atomic Theory
... Atoms are made up of various subatomic particles The three main subatomic particles are distinguishable by mass, charge, and location in the atom ...
... Atoms are made up of various subatomic particles The three main subatomic particles are distinguishable by mass, charge, and location in the atom ...
Atomic Theory Part 1
... The complete atomic symbol‟s mass number‟ (A) and the respective Element‟s „box weight‟ in the periodic table do NOT convey the same information. The complete atomic symbol denotes the mass of ONE isotope of the element in amu, while the p. table gives is the average mass of ALL isotopes of the elem ...
... The complete atomic symbol‟s mass number‟ (A) and the respective Element‟s „box weight‟ in the periodic table do NOT convey the same information. The complete atomic symbol denotes the mass of ONE isotope of the element in amu, while the p. table gives is the average mass of ALL isotopes of the elem ...
Periodic Table Powerpoint
... Horizontal rows Elements from different families Not similar properties Last element an inactive gas Reactive – unreactive 7 periods Valance electrons increase from left to right across a period. ...
... Horizontal rows Elements from different families Not similar properties Last element an inactive gas Reactive – unreactive 7 periods Valance electrons increase from left to right across a period. ...
SCIENCE: EIGHTH GRADE CRT FIRST QUARTER
... Determine the correct electron-dot diagram for various elements. What is the charge of a potassium ion that has 19 protons and 18 electrons? What is the correct formula for sodium chloride? What is the correct formula for calcium fluoride? What is the charge of an ion that contains 16 protons and 18 ...
... Determine the correct electron-dot diagram for various elements. What is the charge of a potassium ion that has 19 protons and 18 electrons? What is the correct formula for sodium chloride? What is the correct formula for calcium fluoride? What is the charge of an ion that contains 16 protons and 18 ...
Atoms
... The element antimony (Sb) has naturally occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
... The element antimony (Sb) has naturally occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
Nuclear Chemistry
... Strong nuclear force holds all nuclei together, but for some isotopes, the force is not enough. These isotopes decay naturally. Isotopes of any atom that can decay are called radioactive. C-14 decays over time at a predictable rate. It’s so dependable that scientists use C-14 dating to determine the ...
... Strong nuclear force holds all nuclei together, but for some isotopes, the force is not enough. These isotopes decay naturally. Isotopes of any atom that can decay are called radioactive. C-14 decays over time at a predictable rate. It’s so dependable that scientists use C-14 dating to determine the ...
Atom/Elements Study Guide
... Protons and neutrons so the charge of the nucleus is positive 3. The atom is composed mostly of empty space. 4. Where is most of the mass of the atom located? In the nucleus 5. How many electrons can exist in the first shell? The second? 2, 8, 8,18 6. Which two subatomic particles have approximatel ...
... Protons and neutrons so the charge of the nucleus is positive 3. The atom is composed mostly of empty space. 4. Where is most of the mass of the atom located? In the nucleus 5. How many electrons can exist in the first shell? The second? 2, 8, 8,18 6. Which two subatomic particles have approximatel ...
ch. 4 atoms outline notes
... (1) In the modern atomic model, electrons can be found only in certain energy levels, not between levels. The location of electrons cannot be predicted precisely. (2) In 1913, Niels Bohr, a Danish physicist, suggested that the energy of each electron was related to the electron’s path around the nuc ...
... (1) In the modern atomic model, electrons can be found only in certain energy levels, not between levels. The location of electrons cannot be predicted precisely. (2) In 1913, Niels Bohr, a Danish physicist, suggested that the energy of each electron was related to the electron’s path around the nuc ...
the atom
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...