ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
04_Lecture Atoms and Elements
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
atom
... All matter is made of atoms Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
... All matter is made of atoms Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
04_Lecture Atoms and Elements
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
UNIT 4 ATOMIC THEORY 1. Atomic theory: Dalton`s model
... ob________ that m______ of the p________ got through the s_____ without turning off, some of them turned off a l________ and a few of them bounced. He d___________ that atoms are al______ e_____ and the po________ ch______ is conc_________ in the ce_______ p____ of the _________ in a v____ _________ ...
... ob________ that m______ of the p________ got through the s_____ without turning off, some of them turned off a l________ and a few of them bounced. He d___________ that atoms are al______ e_____ and the po________ ch______ is conc_________ in the ce_______ p____ of the _________ in a v____ _________ ...
Science Focus 10 Unit 1 Energy and Matter in Chemical
... • Proposed to explain the interaction of chemicals • Key features: • Matter is composed of small indivisible particles (atoms) that can be neither created nor destroyed. • All atoms of the same element are identical in mass and size, but different from atoms of other elements. • Atoms exist in an ot ...
... • Proposed to explain the interaction of chemicals • Key features: • Matter is composed of small indivisible particles (atoms) that can be neither created nor destroyed. • All atoms of the same element are identical in mass and size, but different from atoms of other elements. • Atoms exist in an ot ...
5 - Particles in an atom
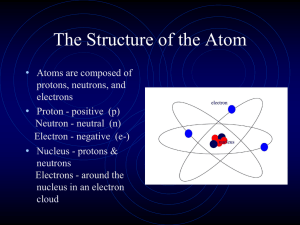
... element. All atoms are basically the same. All atoms of the same element are exactly alike; however, the atoms of a different element will differ from other elements. With the exception of hydrogen, all atoms have three main parts. The parts of an atom are protons, electrons, and neutrons. A proton ...
... element. All atoms are basically the same. All atoms of the same element are exactly alike; however, the atoms of a different element will differ from other elements. With the exception of hydrogen, all atoms have three main parts. The parts of an atom are protons, electrons, and neutrons. A proton ...
The Structure of the Atom - Warren County Public Schools
... Calculate the atomic mass for Carbon: Carbon-12 natural abundance is 98%. Carbon-14 natural abundance is 2%. Carbon’s atomic mass? 12 x 0.98 = 11.76 amu 14 x 0.02 = + 0.28 amu = 12.04 amu * Just remember to convert natural abundance from percentage to decimal form. ...
... Calculate the atomic mass for Carbon: Carbon-12 natural abundance is 98%. Carbon-14 natural abundance is 2%. Carbon’s atomic mass? 12 x 0.98 = 11.76 amu 14 x 0.02 = + 0.28 amu = 12.04 amu * Just remember to convert natural abundance from percentage to decimal form. ...
Chapter 4 Phy. Sci. Atoms 2nd
... Because the numbers of neutrons in the isotopes are different, the mass numbers are also different. • You use the name of the element followed by the mass number of the isotope to identify each isotope: boron10 and boron-11. ...
... Because the numbers of neutrons in the isotopes are different, the mass numbers are also different. • You use the name of the element followed by the mass number of the isotope to identify each isotope: boron10 and boron-11. ...
Study Guide
... electrons were smaller than atoms? •He used a cathode ray tube •Shot a beam of electrons •Deflected the beam with a magnet •Knew how hard the magnet pulled •Saw how far beam was defected •Calculated mass from those numbers ...
... electrons were smaller than atoms? •He used a cathode ray tube •Shot a beam of electrons •Deflected the beam with a magnet •Knew how hard the magnet pulled •Saw how far beam was defected •Calculated mass from those numbers ...
4.1 Experiencing Atoms at Tiburon 4.1 Experiencing Atoms
... • The nuclei of some isotopes of a given element are not stable. • These atoms emit a few energetic subatomic particles from their nuclei and change into different isotopes of different elements. • The emitted subatomic particles are called nuclear radiation. • The isotopes that emit them are te ...
... • The nuclei of some isotopes of a given element are not stable. • These atoms emit a few energetic subatomic particles from their nuclei and change into different isotopes of different elements. • The emitted subatomic particles are called nuclear radiation. • The isotopes that emit them are te ...
atomic number, mass, isotopes
... Lewis dot structures • Lewis dot structures are a way to draw atoms showing only the valence electrons ...
... Lewis dot structures • Lewis dot structures are a way to draw atoms showing only the valence electrons ...
Chapter 4 Atoms and Elements
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
... • When an atom gains or loses electrons, it becomes an ion. • Positively charged ions are called cations. • Negatively charged ions are called anions. • Cations and anions occur together so that matter is chargeneutral. Isotopes: • Atoms of the same element with different numbers of neutrons are cal ...
AP Chemistry Summer Assignment - Belle Vernon Area School District
... as any notes/worksheets from Accel. Chem that you may have. 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge ...
... as any notes/worksheets from Accel. Chem that you may have. 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge ...
Key Concept Summary - Bellingham High School
... mass and 44.4% sulfur by mass. Suppose there is one calcium atom for each sulfur atom in calcium sulfide. Because we know that the mass of a calcium atom relative to that of a sulfur atom must be the same as the mass % in calcium, we know that the ratio of the mass of a calcium atom to that of a sul ...
... mass and 44.4% sulfur by mass. Suppose there is one calcium atom for each sulfur atom in calcium sulfide. Because we know that the mass of a calcium atom relative to that of a sulfur atom must be the same as the mass % in calcium, we know that the ratio of the mass of a calcium atom to that of a sul ...
6.7 Explaining the Periodic Table
... Did you notice any pattern emerging from the periodic table of Bohr–Rutherford diagrams? Mendeleev would have been fascinated to see such a startling pattern from these “family portraits” of the elements. • As you go down each family, the number of electron orbits increases—a new orbit is added wit ...
... Did you notice any pattern emerging from the periodic table of Bohr–Rutherford diagrams? Mendeleev would have been fascinated to see such a startling pattern from these “family portraits” of the elements. • As you go down each family, the number of electron orbits increases—a new orbit is added wit ...
Up And Atom - Lesson Corner
... element are given in each square of the Periodic table: atomic number, atomic mass, chemical symbol and name. The number of protons in an element determines its properties. The number at the top of each square in the table tells how many protons the element has; the atomic number is unique to that e ...
... element are given in each square of the Periodic table: atomic number, atomic mass, chemical symbol and name. The number of protons in an element determines its properties. The number at the top of each square in the table tells how many protons the element has; the atomic number is unique to that e ...
Atom - Malibu High School
... What can you tell me about its protons and electrons? What element has 20 protons? What is the relationship between the # protons and ...
... What can you tell me about its protons and electrons? What element has 20 protons? What is the relationship between the # protons and ...
Document
... ATOMIC NUMBER equals the number of PROTONS or ELECTRONS. Example: Boron, Atomic Number: 5; # of Protons: 5; # of Electrons: 5 ATOMIC MASS equals the number of PROTONS + NEUTRONS. Example: Boron, Atomic Mass: 10.81; # of Protons: 5 + # of Neutrons: 5.81 ...
... ATOMIC NUMBER equals the number of PROTONS or ELECTRONS. Example: Boron, Atomic Number: 5; # of Protons: 5; # of Electrons: 5 ATOMIC MASS equals the number of PROTONS + NEUTRONS. Example: Boron, Atomic Mass: 10.81; # of Protons: 5 + # of Neutrons: 5.81 ...
Half-Life - Chemistry 1 at NSBHS
... Transmutation Reactions • The conversion of an atom of one element to an atom of another element is called transmutation. Transmutation can occur by radioactive decay. Transmutation can also occur when particles bombard the nucleus of an atom. ...
... Transmutation Reactions • The conversion of an atom of one element to an atom of another element is called transmutation. Transmutation can occur by radioactive decay. Transmutation can also occur when particles bombard the nucleus of an atom. ...
notes-part-1
... matter. Chemistry is the study of matter, its properties, and the changes it undergoes. There are two major branches: pure and applied chemistry. Pure chemistry deals with describing known substances and discovering new compounds. Applied chemistry is the search for uses for chemical substances. New ...
... matter. Chemistry is the study of matter, its properties, and the changes it undergoes. There are two major branches: pure and applied chemistry. Pure chemistry deals with describing known substances and discovering new compounds. Applied chemistry is the search for uses for chemical substances. New ...