Unit 1: Atoms, Molecules, and Ions
... Bohr’s model of the atom is very useful for understanding elementary concepts in bonding and chemical reactions However, it is fundamentally flawed and he soon helped replace it with the “Quantum Mechanical Model” of the atom which no longer views the electron as simply a “particle”, but acknowledge ...
... Bohr’s model of the atom is very useful for understanding elementary concepts in bonding and chemical reactions However, it is fundamentally flawed and he soon helped replace it with the “Quantum Mechanical Model” of the atom which no longer views the electron as simply a “particle”, but acknowledge ...
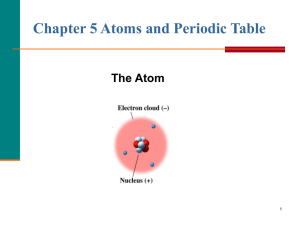
atoms in their elemental state are neutral
... Hints: All atoms in their elemental state are neutral Atomic # = the # p+ in that atom Atoms may contain any or all of the following subatomic particles: protons, neutrons and electrons o protons = +1 charge, weigh 1 atomic mass unit (amu) o electrons = –1 charge, negligible mass o neutrons = ...
... Hints: All atoms in their elemental state are neutral Atomic # = the # p+ in that atom Atoms may contain any or all of the following subatomic particles: protons, neutrons and electrons o protons = +1 charge, weigh 1 atomic mass unit (amu) o electrons = –1 charge, negligible mass o neutrons = ...
Periodic Table for class
... Ionic Bonds = the attractive force between oppositely charged ions that result from the transfer of electrons from one atom to another. If a compound is formed through the transfer of electrons its called Ionic Compound (NaCl) ...
... Ionic Bonds = the attractive force between oppositely charged ions that result from the transfer of electrons from one atom to another. If a compound is formed through the transfer of electrons its called Ionic Compound (NaCl) ...
Periodic Table for class
... Ionic Bonds = the attractive force between oppositely charged ions that result from the transfer of electrons from one atom to another. If a compound is formed through the transfer of electrons its called Ionic Compound (NaCl) ...
... Ionic Bonds = the attractive force between oppositely charged ions that result from the transfer of electrons from one atom to another. If a compound is formed through the transfer of electrons its called Ionic Compound (NaCl) ...
Early Atomic Theory
... Most of the particles passed through the gold foil, but some were deflected and some even bounced back! This suggested the gold atoms must have a densely, positively charged nucleus to affect the path of an α particle (a positively charged He atom). ...
... Most of the particles passed through the gold foil, but some were deflected and some even bounced back! This suggested the gold atoms must have a densely, positively charged nucleus to affect the path of an α particle (a positively charged He atom). ...
Column A
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
Symbols of Elements - Chemistry with Mr. Patmos
... In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
... In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
What is an atomic number and an atomic mass?
... If you know the atomic number of an element, you also know the number of electrons in an atom of that element - they are both the same. They are the same because an atom has neither a positive nor a negative charge. It is neutral. In order for an atom to be neutral, the positive charges of the proto ...
... If you know the atomic number of an element, you also know the number of electrons in an atom of that element - they are both the same. They are the same because an atom has neither a positive nor a negative charge. It is neutral. In order for an atom to be neutral, the positive charges of the proto ...
Notes - Science 2015-2016
... • With a partner at your table, complete the ion worksheet. You will have approximately 10 minutes. • Only talk about the task at hand. I will take points off your grade if you are not! ▫ Once you have decided on an area to work, you may not get out of your seat unless you ask. ...
... • With a partner at your table, complete the ion worksheet. You will have approximately 10 minutes. • Only talk about the task at hand. I will take points off your grade if you are not! ▫ Once you have decided on an area to work, you may not get out of your seat unless you ask. ...
History of the Atom
... All matter is made up of small particles called atoms Atoms cannot be created, destroyed or divided into smaller particles All atoms of the same element are identical in mass and size All atoms of different elements differ from each other in mass and size Compounds are created when atoms o ...
... All matter is made up of small particles called atoms Atoms cannot be created, destroyed or divided into smaller particles All atoms of the same element are identical in mass and size All atoms of different elements differ from each other in mass and size Compounds are created when atoms o ...
Chapter 2
... changing its identity, hence the concept of isotopes. Different isotopes of a given element have the same number of protons, but different numbers of neutrons in the nucleus. Case Study 1: Carbon (Atomic Number 6) All atoms of Carbon will be identified by Atomic Number 6, having this number of proto ...
... changing its identity, hence the concept of isotopes. Different isotopes of a given element have the same number of protons, but different numbers of neutrons in the nucleus. Case Study 1: Carbon (Atomic Number 6) All atoms of Carbon will be identified by Atomic Number 6, having this number of proto ...
PP atoms - Lake County Schools
... Dalton’s Atomic Theory 1. All matter is composed of atoms 2. Atoms of the same element are identical whereas atoms of different elements differ 3. Atoms cannot be subdivided, created, or destroyed 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds 5. In ...
... Dalton’s Atomic Theory 1. All matter is composed of atoms 2. Atoms of the same element are identical whereas atoms of different elements differ 3. Atoms cannot be subdivided, created, or destroyed 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds 5. In ...
Document
... 1. What is the smallest particle something can be divided into? 2. How is an element different from a compound? 3. What does the Atomic Number tell you? 4. What does the Atomic Mass tell you? 5. What are the 3 parts of an atom? ...
... 1. What is the smallest particle something can be divided into? 2. How is an element different from a compound? 3. What does the Atomic Number tell you? 4. What does the Atomic Mass tell you? 5. What are the 3 parts of an atom? ...
Essential Standard: 8.P.1 Understand the properties of matter and
... The distance between molecules in a gas is much larger than that in a solid or a liquid. ...
... The distance between molecules in a gas is much larger than that in a solid or a liquid. ...
Carbon Isotopes
... An electron has an incredibly small mass, which is about 1/2000 the mass of a neutron or a proton. ...
... An electron has an incredibly small mass, which is about 1/2000 the mass of a neutron or a proton. ...
Mileposts on the road to the atom
... of one element combined with the same mass of another element is a simple whole number ...
... of one element combined with the same mass of another element is a simple whole number ...
Praxis II Chemistry prep
... 1. Draw representations of solid, liquid and gas at the atomic level. How are your drawings different? How the same? 1. What happens to a gas volume when it is compressed? What happens to a liquid volume when it is compressed? What happens to a solid volume when it is compressed? 1. What happens to ...
... 1. Draw representations of solid, liquid and gas at the atomic level. How are your drawings different? How the same? 1. What happens to a gas volume when it is compressed? What happens to a liquid volume when it is compressed? What happens to a solid volume when it is compressed? 1. What happens to ...
No Slide Title
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Unit C3, C3.1
... Use the periodic table on the Data Sheet to answer these questions. The table below gives the electronic structures of four elements, W, X, Y and Z. ...
... Use the periodic table on the Data Sheet to answer these questions. The table below gives the electronic structures of four elements, W, X, Y and Z. ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
All of these can affect the rate at which a
... 58. If two covalently bonded atoms are identical, the bond is A coordinate covalent B nonpolar covalent. C polar covalent. D nonionic. 59. A ____ shows the types and numbers of atoms joined in a single molecule of a molecular compound. A ionic bond B molecular formula. C chemical formula. D covalent ...
... 58. If two covalently bonded atoms are identical, the bond is A coordinate covalent B nonpolar covalent. C polar covalent. D nonionic. 59. A ____ shows the types and numbers of atoms joined in a single molecule of a molecular compound. A ionic bond B molecular formula. C chemical formula. D covalent ...
Atoms, and Elements
... If the Houston Astrodome was an atom, a marble placed in the stadium would be the size of the nucleus Most of the mass of the atom is in the nucleus Most of the atom is empty space Atomic number- the number of protons in a nucleus; symbol is Z 1. in a neutral atom, the number of protons is equ ...
... If the Houston Astrodome was an atom, a marble placed in the stadium would be the size of the nucleus Most of the mass of the atom is in the nucleus Most of the atom is empty space Atomic number- the number of protons in a nucleus; symbol is Z 1. in a neutral atom, the number of protons is equ ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.