Heat Transfer: Conduction, Convection and Latent Heat In addition
... Heat Transfer: Conduction, Convection and Latent Heat In addition to radiation, energy can also be transferred in the form of heat. There are three ways this can happen: ...
... Heat Transfer: Conduction, Convection and Latent Heat In addition to radiation, energy can also be transferred in the form of heat. There are three ways this can happen: ...
How Your Body Loses Heat
... evaporation and away from radiation, convection and conduction as the external temperature increases . ...
... evaporation and away from radiation, convection and conduction as the external temperature increases . ...
EML 6154 - UFL MAE - University of Florida
... Office: 310 MAE-A; Phone: 392-0889; E-mail: [email protected] Office Hours: M W F 1:30 to 2:30 PM TA: Sai Tej ([email protected]) Tue. Thu. 1:30 to 2:30 PM Location: MAE-C 130 Text: Heat Conduction, 3rd Edition, D. Hahn and M.N. Ozisik Web: https://lss.at.ufl.edu/ Objectives: The goal of this course is ...
... Office: 310 MAE-A; Phone: 392-0889; E-mail: [email protected] Office Hours: M W F 1:30 to 2:30 PM TA: Sai Tej ([email protected]) Tue. Thu. 1:30 to 2:30 PM Location: MAE-C 130 Text: Heat Conduction, 3rd Edition, D. Hahn and M.N. Ozisik Web: https://lss.at.ufl.edu/ Objectives: The goal of this course is ...
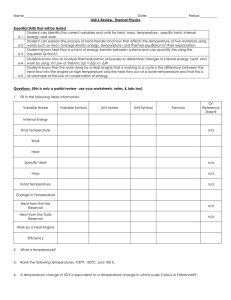
Heat Heat Capacity Latent Heat Latent Heat
... Each of these phase changes requires a certain amount of heat, although the temperature does not change. If a solid becomes liquid, or vice versa, the amount of heat per gram is the latent heat of fusion. If a liquid becomes gas, or vice versa, the amount of heat per gram is the latent heat of ...
... Each of these phase changes requires a certain amount of heat, although the temperature does not change. If a solid becomes liquid, or vice versa, the amount of heat per gram is the latent heat of fusion. If a liquid becomes gas, or vice versa, the amount of heat per gram is the latent heat of ...
Condensation and the Nusselt`s Film Theory
... Condensation occurs if a vapor is cooled below its (pressure dependent) saturation temperature. The heat of evaporation which is released during condensation must be removed by heat transfer, e.g. at a cooled wall. Figure 1 shows how saturated vapor at temperature Ts is condensing on a vertical wall ...
... Condensation occurs if a vapor is cooled below its (pressure dependent) saturation temperature. The heat of evaporation which is released during condensation must be removed by heat transfer, e.g. at a cooled wall. Figure 1 shows how saturated vapor at temperature Ts is condensing on a vertical wall ...
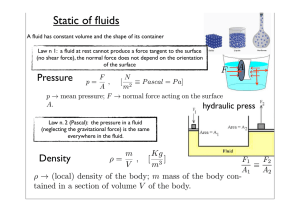
Static of fluids
... or give a quantity Q of heat where m is the mass and cL is the latent heat. ...
... or give a quantity Q of heat where m is the mass and cL is the latent heat. ...
Heat pipe
A heat pipe is a heat-transfer device that combines the principles of both thermal conductivity and phase transition to efficiently manage the transfer of heat between two solid interfaces.At the hot interface of a heat pipe a liquid in contact with a thermally conductive solid surface turns into a vapor by absorbing heat from that surface. The vapor then travels along the heat pipe to the cold interface and condenses back into a liquid - releasing the latent heat. The liquid then returns to the hot interface through either capillary action, centrifugal force, or gravity, and the cycle repeats. Due to the very high heat transfer coefficients for boiling and condensation, heat pipes are highly effective thermal conductors. The effective thermal conductivity varies with heat pipe length, and can approach 7002100000000000000♠100 kW/(m⋅K) for long heat pipes, in comparison with approximately 6999400000000000000♠0.4 kW/(m⋅K) for copper.