Evaporation Technology for Industrial Wastewater Treatment
... boiling under vacuum, while also utilizing the vapor produced within the first boiling stage (effect) to produce new vapor in a next stage at an even lower temperature and pressure. This vapor recycle can be cascaded several times in a (multiple effect) sequence. External heat energy is added only i ...
... boiling under vacuum, while also utilizing the vapor produced within the first boiling stage (effect) to produce new vapor in a next stage at an even lower temperature and pressure. This vapor recycle can be cascaded several times in a (multiple effect) sequence. External heat energy is added only i ...
Heat of Fusion Handout March 2014
... 2. DRYING THE ICE: If the ice is not dried there will be water at 0oC on the ice. The added water will contribute to the final mass of liquid but it will not gain the amount of heat that an equivalent amount of ice would gain. The initial temperature of the water in the calorimeter will not have to ...
... 2. DRYING THE ICE: If the ice is not dried there will be water at 0oC on the ice. The added water will contribute to the final mass of liquid but it will not gain the amount of heat that an equivalent amount of ice would gain. The initial temperature of the water in the calorimeter will not have to ...
Atmosphere
... – About 30oN or 30oS (tropical deserts) • The air subsides with adiabatic warming process, the potential energy is converted to sensible heat, and produce clear cloudless skies. • Most incoming energy is used for heating ground surface which then heats the atmosphere. (sensible heat) • During night, ...
... – About 30oN or 30oS (tropical deserts) • The air subsides with adiabatic warming process, the potential energy is converted to sensible heat, and produce clear cloudless skies. • Most incoming energy is used for heating ground surface which then heats the atmosphere. (sensible heat) • During night, ...
6B.1 THE BASIS FOR THE NEW WIND CHILL TEMPERATURE
... wind speed. The chart was based on the work of Siple and Passel (1945) performed in an Antarctic winter. Subsequently, many researchers concluded that this chart was in error, that it exaggerated the effect of the wind and its WCT’s were too cold. These included Bluestein (1998), Buettner (1952), Bu ...
... wind speed. The chart was based on the work of Siple and Passel (1945) performed in an Antarctic winter. Subsequently, many researchers concluded that this chart was in error, that it exaggerated the effect of the wind and its WCT’s were too cold. These included Bluestein (1998), Buettner (1952), Bu ...
Introduction
... then turned on and set at a desired rate to pump water to the reboiler. Once the reboiler reached the correct level, the feed was turned off and heat was added to start the separation process. Temperature measurements were taken from the reboiler from the beginning (room temp) all the way to steady ...
... then turned on and set at a desired rate to pump water to the reboiler. Once the reboiler reached the correct level, the feed was turned off and heat was added to start the separation process. Temperature measurements were taken from the reboiler from the beginning (room temp) all the way to steady ...
Optimal heating and cooling strategies for heat exchanger design
... be completed in a given, fixed time r. The system has a known heat capacity C and a time-dependent temperature T(t) with required initial and final temperatures T(0) and T(r), respectively. Heating or cooling is effected from an external reservoir of known heat capacity C, and adjustable time-depend ...
... be completed in a given, fixed time r. The system has a known heat capacity C and a time-dependent temperature T(t) with required initial and final temperatures T(0) and T(r), respectively. Heating or cooling is effected from an external reservoir of known heat capacity C, and adjustable time-depend ...
29-Thermal Exoskeleton - European School Luxembourg
... the disruption in the normal function of thermoregulation systems in an organism occur, or the conditions are too severe to prevent complications, a person becomes at risk of hyperthermia or hypothermia. In those cases, body temperatures that increase beyond 38°C, or drop below 35°C, pose increasing ...
... the disruption in the normal function of thermoregulation systems in an organism occur, or the conditions are too severe to prevent complications, a person becomes at risk of hyperthermia or hypothermia. In those cases, body temperatures that increase beyond 38°C, or drop below 35°C, pose increasing ...
Procedure
... between the amount of heat in a system in the final state Hf and the amount of heat in a system in the initial state Hi. H = Hf – Hi If heat is given off during a reaction, then there will be less enthalpy in the system in the final state. This means that Hf will be smaller than Hi and, therefore, ...
... between the amount of heat in a system in the final state Hf and the amount of heat in a system in the initial state Hi. H = Hf – Hi If heat is given off during a reaction, then there will be less enthalpy in the system in the final state. This means that Hf will be smaller than Hi and, therefore, ...
Chapter 18
... Is used to cook foods with a certain amount of moisture, the moisture might be a stock, sauce, or custard Baked products are often cooked covered to keep the moisture in the product Both baking and roasting use a combination of convection and radiation to transfer heat to foods Some require a gentle ...
... Is used to cook foods with a certain amount of moisture, the moisture might be a stock, sauce, or custard Baked products are often cooked covered to keep the moisture in the product Both baking and roasting use a combination of convection and radiation to transfer heat to foods Some require a gentle ...
Aalborg Universitet Heiselberg, Per Kvols
... An evaporative layer to be applied on the inner surfaces of outer walls has been designed and proposed in this study to reduce the cooling load of buildings in Mediterranean region. This can be considered as a new approach. A building has been considered in Mediterranean region as the case study. Co ...
... An evaporative layer to be applied on the inner surfaces of outer walls has been designed and proposed in this study to reduce the cooling load of buildings in Mediterranean region. This can be considered as a new approach. A building has been considered in Mediterranean region as the case study. Co ...
Experiment 5
... where L is the latent heat of transformation appropriate for the type of phase change taking place. In using eq. 2, the sign must be chosen appropriately according to the sign convention discussed above. As an example, if the object is melting, heat is being added so that the plus sign is correct. T ...
... where L is the latent heat of transformation appropriate for the type of phase change taking place. In using eq. 2, the sign must be chosen appropriately according to the sign convention discussed above. As an example, if the object is melting, heat is being added so that the plus sign is correct. T ...
Chapter 5, Problem 1
... A double-pane window consists of two 3-mm thick layers of glass separated by a 12-mm wide stagnant air space. For specified indoors and outdoors temperatures, the rate of heat loss through the window and the inner surface temperature of the window are to be determined. Assumptions 1 Heat transfer th ...
... A double-pane window consists of two 3-mm thick layers of glass separated by a 12-mm wide stagnant air space. For specified indoors and outdoors temperatures, the rate of heat loss through the window and the inner surface temperature of the window are to be determined. Assumptions 1 Heat transfer th ...
Temperature Coefficient of Resistance
... that both directly and indirectly affect our daily lives, the world as we know it would be out-ofcontrol in a scientific sense. Not all great innovations in feedback control, however, were designed by man. The natural world, left untouched from human design, exhibits some of the most efficient use o ...
... that both directly and indirectly affect our daily lives, the world as we know it would be out-ofcontrol in a scientific sense. Not all great innovations in feedback control, however, were designed by man. The natural world, left untouched from human design, exhibits some of the most efficient use o ...
Document
... do not coincide with the months receiving the most or lowest radiation. The other processes which also control temperature (i.e. winds and surface ocean currents do not happen instantaneously. There is a “lag time”. ...
... do not coincide with the months receiving the most or lowest radiation. The other processes which also control temperature (i.e. winds and surface ocean currents do not happen instantaneously. There is a “lag time”. ...
Specific Heat of Copper
... A 25kg cylinder of copper is heated from room temerature. The same 374kJ of thermal energy were used during the heating process but this time the copper’s temperature rose from room temperature to only 58.4ºC. Calculate the specific heat capacity of this piece of copper. Answer: 390J Solving for ...
... A 25kg cylinder of copper is heated from room temerature. The same 374kJ of thermal energy were used during the heating process but this time the copper’s temperature rose from room temperature to only 58.4ºC. Calculate the specific heat capacity of this piece of copper. Answer: 390J Solving for ...
AIR CONDITIONING
... Warm air holds moisture. For example, your body produces perspiration when heated. If it is warm, and the humidity is high, the hot, humid air cannot absorb any excess moisture, and perspiration remains on the skin. If it is cooler, and the humidity is low, the air can absorb moisture from the skin ...
... Warm air holds moisture. For example, your body produces perspiration when heated. If it is warm, and the humidity is high, the hot, humid air cannot absorb any excess moisture, and perspiration remains on the skin. If it is cooler, and the humidity is low, the air can absorb moisture from the skin ...
Specific Heat and Phase Change - CK
... • The amount of heat capacitance (and thus its specific heat value) is related to something called ’degrees of freedom,’ which basically says how free is the object to move in different ways (and thus how much kinetic energy can it store inside itself without breaking apart). For example, solids hav ...
... • The amount of heat capacitance (and thus its specific heat value) is related to something called ’degrees of freedom,’ which basically says how free is the object to move in different ways (and thus how much kinetic energy can it store inside itself without breaking apart). For example, solids hav ...
WS Specific Heat 2
... WS Specific Heat 2 1. How much heat is required to raise the temperature of 19.68 g of calcium from 18.00 °C to 82.40 °C? The specific heat of calcium is 0.647 J/g°C. 2. 400.0 J of heat are applied to a sample of beryllium. Its temperature increases from 22.00 °C to 50.00 °C. What is the sample’s ma ...
... WS Specific Heat 2 1. How much heat is required to raise the temperature of 19.68 g of calcium from 18.00 °C to 82.40 °C? The specific heat of calcium is 0.647 J/g°C. 2. 400.0 J of heat are applied to a sample of beryllium. Its temperature increases from 22.00 °C to 50.00 °C. What is the sample’s ma ...
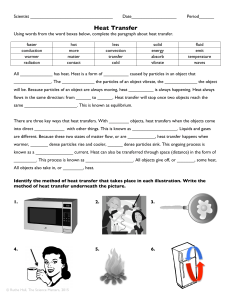
Heat Transfer - Granville County Public Schools
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
Modelling of wire die coating
... exchange between outer stream which could be waste dirty water and inner stream. The OPHE are often used for heat recovery purposes in laundries, paper industries etc. The main problem in thermo dynamical calculation of such type of heat exchanger is determination of the heat transfer coefficient on ...
... exchange between outer stream which could be waste dirty water and inner stream. The OPHE are often used for heat recovery purposes in laundries, paper industries etc. The main problem in thermo dynamical calculation of such type of heat exchanger is determination of the heat transfer coefficient on ...
Q equations.notebook
... • Where does this energy go? > Particles must overcome forces of attraction to move farther apart during phase change (s → l) ...
... • Where does this energy go? > Particles must overcome forces of attraction to move farther apart during phase change (s → l) ...
Solution
... 15.) A CD player and its battery together do 500 kJ of work, and the battery also releases 250 kJ of energy as heat and the CD player releases 50 kJ as heat due to friction from spinning. What is the change in internal energy (kJ) of the system, with the system regarded as the battery and CD player ...
... 15.) A CD player and its battery together do 500 kJ of work, and the battery also releases 250 kJ of energy as heat and the CD player releases 50 kJ as heat due to friction from spinning. What is the change in internal energy (kJ) of the system, with the system regarded as the battery and CD player ...
Summary
... you think the rate of heat transfer from the surface has increased or decreased as a result of addition of fins? 5. Fins are normally meant to enhance heat transfer. Under what circumstances the addition of fins may actually decrease heat transfer? 6. Hot water is to be cooled as it flows through th ...
... you think the rate of heat transfer from the surface has increased or decreased as a result of addition of fins? 5. Fins are normally meant to enhance heat transfer. Under what circumstances the addition of fins may actually decrease heat transfer? 6. Hot water is to be cooled as it flows through th ...
CHEMISTRY 3310 PROBLEM SHEET #4 1. The specific heats of a
... 25. Predict the enthalpy of reaction for the oxidation of CH3OH (l) CH3OH (l) + 3/2 O2 (g) CO2 (g) + 2 H2O (l) using the given table of mean bond energies. The enthalpy of vaporization at 25°C is 37.99 kJ/mole for CH3OH and 44.011 kJ/mole for H2O. 26. For the constant pressure process, C2H6 ...
... 25. Predict the enthalpy of reaction for the oxidation of CH3OH (l) CH3OH (l) + 3/2 O2 (g) CO2 (g) + 2 H2O (l) using the given table of mean bond energies. The enthalpy of vaporization at 25°C is 37.99 kJ/mole for CH3OH and 44.011 kJ/mole for H2O. 26. For the constant pressure process, C2H6 ...
Intercooler

An intercooler is any mechanical device used to cool a fluid, including liquids or gases, between stages of a multi-stage heating process, typically a heat exchanger that removes waste heat in a gas compressor. They are used in many applications, including air compressors, air conditioners, refrigerators, and gas turbines, and are widely known in automotive use as an air-to-air or air-to-liquid cooler for forced induction (turbocharged or supercharged) internal combustion engines to improve their volumetric efficiency by increasing intake air charge density through nearly isobaric (constant pressure) cooling.