Depressants - White Ribbon Association
... depressant drugs have been linked with irritability, paranoia and even suicidal thoughts. There is also a high risk of addiction with depressant drugs, and the brain can soon begin to crave more and more. Continued heavy use can lead to overdosing and possibly death. ...
... depressant drugs have been linked with irritability, paranoia and even suicidal thoughts. There is also a high risk of addiction with depressant drugs, and the brain can soon begin to crave more and more. Continued heavy use can lead to overdosing and possibly death. ...
Statistics on pharmaceuticals 2015
... control pills, contraceptive patch and vaginal ring. The use of short-acting contraceptives has in general declined since 2011 while long-acting has increased. Approximately 40 percent of women aged 20 ̶ 24 purchased at least one short-acting contraceptive in 2015, compared to nearly 50 percent in 2 ...
... control pills, contraceptive patch and vaginal ring. The use of short-acting contraceptives has in general declined since 2011 while long-acting has increased. Approximately 40 percent of women aged 20 ̶ 24 purchased at least one short-acting contraceptive in 2015, compared to nearly 50 percent in 2 ...
nations unies
... pharmaceuticals will undoubtedly change with time and therefore an assessment performed now may not be applicable in the future. HSAC will revisit this document as required. ...
... pharmaceuticals will undoubtedly change with time and therefore an assessment performed now may not be applicable in the future. HSAC will revisit this document as required. ...
Pharmaceuticals and OTC*s
... The FDA allows new medicines to be used only if they work and if they are safe enough. When a medicine's benefits outweigh its known risks, the FDA usually approves the sale of the drug. The FDA can withdraw a medication from the market at any time if it later is found to cause harmful side effects. ...
... The FDA allows new medicines to be used only if they work and if they are safe enough. When a medicine's benefits outweigh its known risks, the FDA usually approves the sale of the drug. The FDA can withdraw a medication from the market at any time if it later is found to cause harmful side effects. ...
Pharmaceutical Manufactures Formulary and Supplemental
... • Authorizes the Agency to negotiate rebates from manufacturers in addition to those required by Title XIX of the Social Security Act • No less than 10 percent of Average Manufacturer Price (as defined in 42 U.S.C. s. 1936); or federal or supplemental rebate, or both, equals or exceeds 25 percent • ...
... • Authorizes the Agency to negotiate rebates from manufacturers in addition to those required by Title XIX of the Social Security Act • No less than 10 percent of Average Manufacturer Price (as defined in 42 U.S.C. s. 1936); or federal or supplemental rebate, or both, equals or exceeds 25 percent • ...
Drug Discovery and Development
... • The route must be suitable to the “scale up” needed for the production of at least tens of kilograms of final product • This may limit the structural complexity and/or ultimate size (i.e. mw) of the final product • In some cases, it may be useful to design microbial processes which produce highly ...
... • The route must be suitable to the “scale up” needed for the production of at least tens of kilograms of final product • This may limit the structural complexity and/or ultimate size (i.e. mw) of the final product • In some cases, it may be useful to design microbial processes which produce highly ...
Pharmacology 1 for pharmacy students
... purely basic sciences and clinical sciences to promote a safe and effective drug use optimizing benefits and minimizing risks. Rational drug use embraces not only rational drug prescribing by the clinical practitioner but also rational drug dispensing by the pharmacist and rational drug consumption ...
... purely basic sciences and clinical sciences to promote a safe and effective drug use optimizing benefits and minimizing risks. Rational drug use embraces not only rational drug prescribing by the clinical practitioner but also rational drug dispensing by the pharmacist and rational drug consumption ...
7: Efforts to Improve Drug Information In Developing Countries
... In addition to labeling provided with a drug, compendia of pharmaceutical information from industrialized countries are useful sources of prescribing information for officials and physicians. WHO is working to provide national drug regulatory authorities in developing countries with three of these c ...
... In addition to labeling provided with a drug, compendia of pharmaceutical information from industrialized countries are useful sources of prescribing information for officials and physicians. WHO is working to provide national drug regulatory authorities in developing countries with three of these c ...
ONO Announces Results from Phase 1/2 and Phase 3 Clinical
... The results showed that the percentage of patients achieving serum iPTH management level within 60-240 pg/mL (recommended by JSDT guideline*) at 85 days after the first dose of study drug, the primary endpoint, was significantly higher in the etelcalcetide group (59.0%) than in the placebo group (1. ...
... The results showed that the percentage of patients achieving serum iPTH management level within 60-240 pg/mL (recommended by JSDT guideline*) at 85 days after the first dose of study drug, the primary endpoint, was significantly higher in the etelcalcetide group (59.0%) than in the placebo group (1. ...
Document
... Pain relief Rapid development of dependence Lethargy and weight loss Loss of sexual drive ...
... Pain relief Rapid development of dependence Lethargy and weight loss Loss of sexual drive ...
0302320.01 - American Bar Association
... of the existing Commissioner of Food and Drugs, Dr. Jane Henney. Since that date in early 2001, the U.S. Food and Drug Administration (FDA) has been without a Commissioner to lead it. The White House has nominated no candidate for this important public position in nearly 20 months in office; one-hal ...
... of the existing Commissioner of Food and Drugs, Dr. Jane Henney. Since that date in early 2001, the U.S. Food and Drug Administration (FDA) has been without a Commissioner to lead it. The White House has nominated no candidate for this important public position in nearly 20 months in office; one-hal ...
Designer and look
... In 1982 the Food and Drug Administration (FDA) banned the sale of the triple combination of caffeine, ephedrine and phenylpropanolamine. This federal and related state statues prohibited the marketing of these look-alikes whose packaging appeared to look like amphetamines. The drug industry countere ...
... In 1982 the Food and Drug Administration (FDA) banned the sale of the triple combination of caffeine, ephedrine and phenylpropanolamine. This federal and related state statues prohibited the marketing of these look-alikes whose packaging appeared to look like amphetamines. The drug industry countere ...
Date - Skills Commons
... 65. Companies that administer drug benefit programs are called A) co-insurance. B) patient assistance programs. C) pharmacy benefit managers. D) workers' relief programs. ...
... 65. Companies that administer drug benefit programs are called A) co-insurance. B) patient assistance programs. C) pharmacy benefit managers. D) workers' relief programs. ...
Intro to IMC
... • Advertising is any paid form of nonpersonal presentation and promotion of ideas, goods and services by an identified ...
... • Advertising is any paid form of nonpersonal presentation and promotion of ideas, goods and services by an identified ...
Living better through chemistry: dementia, long
... • LTC homes are not covered under the provisions of Medicare & therefore federal government does not contribute any money for them and therefore lacks any method of enforcing legislation • Care in LTC homes is part of the delivery of health care and is therefore a provincial responsibility • No prov ...
... • LTC homes are not covered under the provisions of Medicare & therefore federal government does not contribute any money for them and therefore lacks any method of enforcing legislation • Care in LTC homes is part of the delivery of health care and is therefore a provincial responsibility • No prov ...
Names
... Herbal medicine is a form of alternative/complementary medicine Herbal products are available without a prescription Remedies can be made from roots, barks, leaves, fruits, berries, and flowers. Some are effective Some are not effective Some are harmful ...
... Herbal medicine is a form of alternative/complementary medicine Herbal products are available without a prescription Remedies can be made from roots, barks, leaves, fruits, berries, and flowers. Some are effective Some are not effective Some are harmful ...
Published 2 September 2008, doi:10
... and colleagues (doi: 10.1136/bmj.a1055), provides compelling evidence that this does have consequences for public health and of the need for better regulatory oversight.1 Direct to consumer advertising from the United States reaches Canadians via cable television and US magazines sold in Canada. Law ...
... and colleagues (doi: 10.1136/bmj.a1055), provides compelling evidence that this does have consequences for public health and of the need for better regulatory oversight.1 Direct to consumer advertising from the United States reaches Canadians via cable television and US magazines sold in Canada. Law ...
ROVI announces the marketing agreement of
... Orexigen Therapeutics, Inc. is a biopharmaceutical company focused on the treatment of obesity. Orexigen's first product, Contrave® (naltrexone HCl and bupropion HCl extended release), was approved in the United States in September 2014 and became the most prescribed branded obesity medication in th ...
... Orexigen Therapeutics, Inc. is a biopharmaceutical company focused on the treatment of obesity. Orexigen's first product, Contrave® (naltrexone HCl and bupropion HCl extended release), was approved in the United States in September 2014 and became the most prescribed branded obesity medication in th ...
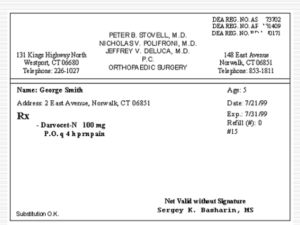
Prescriptions – Chapter 5
... Once prescription information is finalized, a label and receipt is printed. The pharmacy tech completes the fill process by placing the correct amount of medication into an appropriate container and applies the label. The pharmacist then checks the final product and the label. ...
... Once prescription information is finalized, a label and receipt is printed. The pharmacy tech completes the fill process by placing the correct amount of medication into an appropriate container and applies the label. The pharmacist then checks the final product and the label. ...