ISN III: Building Atoms and Organizing Matter
... 11.Hydrogen has the atomic number 1 because it has ________ proton. It’s symbol is ________. 12. _____________ electrons can fit in the first shell. ____________ electrons fit in the 2nd and 3rd shell. 13.______________________ have the same atomic number but different atomic mass and mass number. 1 ...
... 11.Hydrogen has the atomic number 1 because it has ________ proton. It’s symbol is ________. 12. _____________ electrons can fit in the first shell. ____________ electrons fit in the 2nd and 3rd shell. 13.______________________ have the same atomic number but different atomic mass and mass number. 1 ...
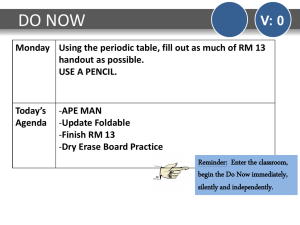
DO NOW - PBworks
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
File - Biochemistry
... Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic masses and relative abundances as follows: 23.985 amu (78.99%) 24.986 amu (10.00%) 25.982 amu (11.01%) ...
... Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic masses and relative abundances as follows: 23.985 amu (78.99%) 24.986 amu (10.00%) 25.982 amu (11.01%) ...
Document
... A scientific problem. A periodic pattern to be discovered. Two detectives. One answer found and reformulated. Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were ...
... A scientific problem. A periodic pattern to be discovered. Two detectives. One answer found and reformulated. Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were ...
Build an Atom - Sterlingwikisci
... 4. An atom with the same number of protons and electrons has a charge of _________. 5. Adding two electrons to a neutral atom produces an ion with a charge of _________. 6. An atom with six protons and five electrons would have a charge of _________. 7. What atom is created with nine protons, nine n ...
... 4. An atom with the same number of protons and electrons has a charge of _________. 5. Adding two electrons to a neutral atom produces an ion with a charge of _________. 6. An atom with six protons and five electrons would have a charge of _________. 7. What atom is created with nine protons, nine n ...
Chapter 4 Quiz ____ 1. The Greek philosopher Democritus coined
... ____ 2. Which of the following is NOT part of John Dalton’s atomic theory? a. All elements are composed of atoms. b. All atoms of the same element have the same mass. c. Atoms contain subatomic particles. d. A compound contains atoms of more than one element. ____ 3. Rutherford’s gold foil experimen ...
... ____ 2. Which of the following is NOT part of John Dalton’s atomic theory? a. All elements are composed of atoms. b. All atoms of the same element have the same mass. c. Atoms contain subatomic particles. d. A compound contains atoms of more than one element. ____ 3. Rutherford’s gold foil experimen ...
Periodic Table Properties Notes s1
... gained electrons and has a negative or positive charge. • Atoms that lose electrons have a positive charge. Atoms that gain electrons have a negative charge. • What ion an atom will form can be determined by its group on the periodic table. ...
... gained electrons and has a negative or positive charge. • Atoms that lose electrons have a positive charge. Atoms that gain electrons have a negative charge. • What ion an atom will form can be determined by its group on the periodic table. ...
Chem - Humble ISD
... Not exactly the same as the number of neutrons + number of protons because of isotopes (see next page). For our ID purposes, round off to the nearest ______________________. Number of neutrons = mass minus atomic number (big # - little #) i.e. Number of neutrons = Remember, normal single atoms are e ...
... Not exactly the same as the number of neutrons + number of protons because of isotopes (see next page). For our ID purposes, round off to the nearest ______________________. Number of neutrons = mass minus atomic number (big # - little #) i.e. Number of neutrons = Remember, normal single atoms are e ...
Atomic Theory Practice Test
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
2.1 Atoms and Bonds
... A chemical change occurs when compounds are formed Reactants are particles that are present before the reaction Products are particles that are present after the reaction Of the form: Reactant Products ◦ Ex: 2H2 + O2 2H2O ...
... A chemical change occurs when compounds are formed Reactants are particles that are present before the reaction Products are particles that are present after the reaction Of the form: Reactant Products ◦ Ex: 2H2 + O2 2H2O ...
Regents Chemistry
... Robert Boyle (1627 – 1691) – the first scientist to recognize the importance of careful measurements. Defined the term element in terms of experimentation; a substance was an element unless it could be broken down into two or more simpler substances ...
... Robert Boyle (1627 – 1691) – the first scientist to recognize the importance of careful measurements. Defined the term element in terms of experimentation; a substance was an element unless it could be broken down into two or more simpler substances ...
File
... a) Group IA - alkali metals (Li, Na, K excluding hydrogen which is a non-metal) - Highly reactive metals meaning they combine easily with other elements. - Have one electron in the highest energy level of their atoms thus it is easily lost. Consequently, alkali metals are found in nature as positive ...
... a) Group IA - alkali metals (Li, Na, K excluding hydrogen which is a non-metal) - Highly reactive metals meaning they combine easily with other elements. - Have one electron in the highest energy level of their atoms thus it is easily lost. Consequently, alkali metals are found in nature as positive ...
"The Atom" Guided Notes
... Bohr Model Bohr discovered that ________________________________ move in orbitals _______________________the nucleus of an atom. Although, we don’t know exactly where electrons are at any given time, we now have a model that helps scientists estimate where electrons are likely to be located at any g ...
... Bohr Model Bohr discovered that ________________________________ move in orbitals _______________________the nucleus of an atom. Although, we don’t know exactly where electrons are at any given time, we now have a model that helps scientists estimate where electrons are likely to be located at any g ...
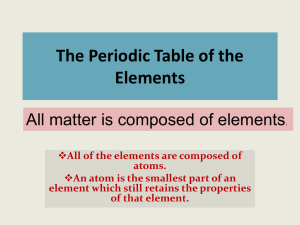
The Periodic Table of the Elements
... element is determined by its periodic table group (vertical column) in which the element is categorized. FOR THE “A” GROUPS ONLY, the number of the group identifies how many valence electrons are contained within the elements listed under that particular column. ...
... element is determined by its periodic table group (vertical column) in which the element is categorized. FOR THE “A” GROUPS ONLY, the number of the group identifies how many valence electrons are contained within the elements listed under that particular column. ...
worksheet #1 - chemistryrocks.net
... number of ______________________ in a neutral atom of that element. The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the ______________________ atomic number. [4] The ______________________ of an element is the av ...
... number of ______________________ in a neutral atom of that element. The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the ______________________ atomic number. [4] The ______________________ of an element is the av ...
Name Date ______ Period ______ Chapter 5: Periodic Table
... 7. __________________________ are elements that are poor conductors of heat and electric current. 8. Nonmetals have _______________ points – many nonmetals are ___________ at room temperature. 9. Nonmetals that are solids at room temperature tend to be brittle. If they are hit with a hammer, they s ...
... 7. __________________________ are elements that are poor conductors of heat and electric current. 8. Nonmetals have _______________ points – many nonmetals are ___________ at room temperature. 9. Nonmetals that are solids at room temperature tend to be brittle. If they are hit with a hammer, they s ...
Name the three parts of an atom and where they are located
... What is the atomic mass? The mass of an atom; the # protons + # of neutrons What parts of the atom account for the atomic mass? protons & neutrons What is an isotope? An atom that has the same number of protons but a different number of neutrons Are isotopes always the same element? Why? Yes, the # ...
... What is the atomic mass? The mass of an atom; the # protons + # of neutrons What parts of the atom account for the atomic mass? protons & neutrons What is an isotope? An atom that has the same number of protons but a different number of neutrons Are isotopes always the same element? Why? Yes, the # ...
Atoms, Molecules and Ions
... • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms of the same ...
... • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms of the same ...
Section 6.2 Notes - oologah.k12.ok.us
... Despite their small size, individual atoms are observable with instruments such as scanning tunneling microscopes. By using this microscope, individual atoms can be moved around and arranged in patterns This technology is important in future applications in medicine, communications, solar ener ...
... Despite their small size, individual atoms are observable with instruments such as scanning tunneling microscopes. By using this microscope, individual atoms can be moved around and arranged in patterns This technology is important in future applications in medicine, communications, solar ener ...
Name: Date: ______ Period: _____ Chemistry 1st semester final
... 37. How are the elements arranged on the periodic table?By increasing atomic number 38. Why do element in the same family have similar chemical properties?b/c they have the same number of valance electrons 39. What type of elements is found in the upper right hand part of the periodic table?nonmetal ...
... 37. How are the elements arranged on the periodic table?By increasing atomic number 38. Why do element in the same family have similar chemical properties?b/c they have the same number of valance electrons 39. What type of elements is found in the upper right hand part of the periodic table?nonmetal ...
Unit 5: Atoms and the Periodic Table
... units called atoms, but was unable to provide evidence. ...
... units called atoms, but was unable to provide evidence. ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... (b) the alkali metal with only 2p and 3p electrons ...
... (b) the alkali metal with only 2p and 3p electrons ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... Elements in compounds combine in fixed percentages by mass not volume. 2) Which of the following statements is correct? a) The number of protons and neutrons in the nucleus of an atom are always equal. b) The mass of an atom is contained primarily in the nucleus and the volume of an atom is primaril ...
... Elements in compounds combine in fixed percentages by mass not volume. 2) Which of the following statements is correct? a) The number of protons and neutrons in the nucleus of an atom are always equal. b) The mass of an atom is contained primarily in the nucleus and the volume of an atom is primaril ...
File
... When white light is shone through a prism, a full rainbow of colours is seen. When light produced by hydrogen is examined in the same way, only a few lines of colour are seen. Most colours are missing. ...
... When white light is shone through a prism, a full rainbow of colours is seen. When light produced by hydrogen is examined in the same way, only a few lines of colour are seen. Most colours are missing. ...