Ions and Ionic Compounds
... The Modern View of Atomic Structure The atom consists of positive, negative, and neutral entities (protons, electrons, and neutrons). Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus. There can be a variable number of ne ...
... The Modern View of Atomic Structure The atom consists of positive, negative, and neutral entities (protons, electrons, and neutrons). Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus. There can be a variable number of ne ...
Final Exam - Seattle Central College
... – Recognize that London forces increase with more electrons—use size to determine relative number of electrons for different molecules. – Know the terms: evaporation, boiling point, vapor pressure, volatile, nonvolatile – Recognize how IMF’s influence vapor pressure and boiling point. – Given differ ...
... – Recognize that London forces increase with more electrons—use size to determine relative number of electrons for different molecules. – Know the terms: evaporation, boiling point, vapor pressure, volatile, nonvolatile – Recognize how IMF’s influence vapor pressure and boiling point. – Given differ ...
Unit 2- The Atom
... This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles t ...
... This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles t ...
Unit 2- The Atom
... This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles t ...
... This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles t ...
Module 3: Physical Science
... Elements are the simplest forms of matter. They can exist alone or in various combinations. Different elements can chemically combine to form molecules or molecular compounds. For example, water is a compound, consisting of water molecules. These molecules can be decomposed into the elements hydroge ...
... Elements are the simplest forms of matter. They can exist alone or in various combinations. Different elements can chemically combine to form molecules or molecular compounds. For example, water is a compound, consisting of water molecules. These molecules can be decomposed into the elements hydroge ...
Chemistry: Matter and Change
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
Atomic Number
... All atoms of lithium (left) contain three protons, and all atoms of carbon (right) contain six protons. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake ...
... All atoms of lithium (left) contain three protons, and all atoms of carbon (right) contain six protons. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake ...
Atomic Structure PowerPoint Presentation
... the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is calculated by adding the % of 1H mass foun ...
... the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is calculated by adding the % of 1H mass foun ...
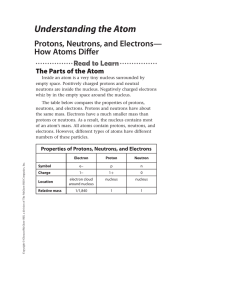
Understanding the Atom
... Look at the periodic table on the inside back cover of this book. Notice that more than 115 different elements have been identified. Recall that an element is a substance made from atoms that all have the same number of protons. For example, the element carbon is made from atoms that all have six pr ...
... Look at the periodic table on the inside back cover of this book. Notice that more than 115 different elements have been identified. Recall that an element is a substance made from atoms that all have the same number of protons. For example, the element carbon is made from atoms that all have six pr ...
Energy and Matter in Chemical Change Science 10
... • Each horizontal row is called a period – Grouped together because of energy levels – Numbered 1-7 ...
... • Each horizontal row is called a period – Grouped together because of energy levels – Numbered 1-7 ...
Lesson Overview
... Some isotopes are radioactive, meaning that their nuclei are unstable and break down at a constant rate over time. Geologists can determine the ages of rocks and fossils by analyzing the isotopes found in them. Radiation from certain isotopes can be used to detect and treat cancer and to kill bacter ...
... Some isotopes are radioactive, meaning that their nuclei are unstable and break down at a constant rate over time. Geologists can determine the ages of rocks and fossils by analyzing the isotopes found in them. Radiation from certain isotopes can be used to detect and treat cancer and to kill bacter ...
chemistry in the 8th grade
... This is a modern version of the periodic table that Mendeleyev came up with. The vertical columns are called groups or families. Elements in these groups would have similar chemical properties. For example, all of the elements in VIIIA have filled outer shells – the maximum number of electrons that ...
... This is a modern version of the periodic table that Mendeleyev came up with. The vertical columns are called groups or families. Elements in these groups would have similar chemical properties. For example, all of the elements in VIIIA have filled outer shells – the maximum number of electrons that ...
Chemistry: Matter and Change
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
Solutions 1a (suggested problems before Exam #1) Chem151 [Kua
... Molecular pictures must show that atoms of every element are conserved. The solid-gas transformation is correctly represented, but the number of molecules is not conserved. Here is one way to show a correct picture: ...
... Molecular pictures must show that atoms of every element are conserved. The solid-gas transformation is correctly represented, but the number of molecules is not conserved. Here is one way to show a correct picture: ...
Chemistry Mid-Term Review Guide
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
04 Atom notes
... How was John Dalton able to study atoms even though he was unable to see them directly? What evidence did he use to form his theory? ___________________________________________________________________ ___________________________________________________________________ List two reasons why the ideas ...
... How was John Dalton able to study atoms even though he was unable to see them directly? What evidence did he use to form his theory? ___________________________________________________________________ ___________________________________________________________________ List two reasons why the ideas ...
Science Focus 9 Matter and Chemical Change Class Notes Topic 1
... elements repeated at periodic intervals. This enabled him to group elements into families. The gaps he left in the organization of the elements in his table were filled in many years later when more elements were discovered. In 1875 gallium was discovered and proved that Mendeleev’s organization of ...
... elements repeated at periodic intervals. This enabled him to group elements into families. The gaps he left in the organization of the elements in his table were filled in many years later when more elements were discovered. In 1875 gallium was discovered and proved that Mendeleev’s organization of ...
Atoms, Elements, and
... you build a full-sized boat and hope it would float? It would be more efficient, less expensive, and safer to build and test a smaller version first. Then, if it didn’t float, you could change your design and build another model. You could keep trying until the model worked. In the case of atoms, sc ...
... you build a full-sized boat and hope it would float? It would be more efficient, less expensive, and safer to build and test a smaller version first. Then, if it didn’t float, you could change your design and build another model. You could keep trying until the model worked. In the case of atoms, sc ...
Chapter42015.1 STUDENT
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.