pwpt chemistry1
... Subscript after a symbol tell the number of atoms of each element H20 has 2 atoms of hydrogen & 1 atom of oxygen Coefficients before a formula tell the number of molecules 3O2 represents 3 molecules of oxygen or (3x2) or 6 atoms of oxygen ...
... Subscript after a symbol tell the number of atoms of each element H20 has 2 atoms of hydrogen & 1 atom of oxygen Coefficients before a formula tell the number of molecules 3O2 represents 3 molecules of oxygen or (3x2) or 6 atoms of oxygen ...
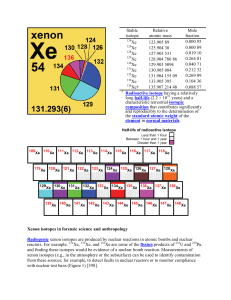
Xenon isotopes in forensic science and anthropology Radiogenic
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
Chapter 4 - Arrangement of Electrons in Atoms
... 1. Electrons have wavelike properties 2. Consider the electron as a wave confined to a space that can have only certain frequencies B. The Heisenbery Uncertainty Principle (Werner Heisenberg - 1927) 1. "It is impossible to determine simultaneously both the position and velocity of an electron or any ...
... 1. Electrons have wavelike properties 2. Consider the electron as a wave confined to a space that can have only certain frequencies B. The Heisenbery Uncertainty Principle (Werner Heisenberg - 1927) 1. "It is impossible to determine simultaneously both the position and velocity of an electron or any ...
Quarks, Leptons, Bosons the LHC and All That
... • It “gives mass” to the fundamental particles (if, in fact, it exists) Without the Higgs (or something) the theory requires that all these fundamental particles have M=0. But we know that’s not the case. ...
... • It “gives mass” to the fundamental particles (if, in fact, it exists) Without the Higgs (or something) the theory requires that all these fundamental particles have M=0. But we know that’s not the case. ...
Introduction - High Energy Physics Group
... Qualitatively: the propagator is inversely proportional to how far the particle is off-shell. The further off-shell, the smaller the probability of producing such a virtual state. ...
... Qualitatively: the propagator is inversely proportional to how far the particle is off-shell. The further off-shell, the smaller the probability of producing such a virtual state. ...
解答六 10.49. Model: Since there is no friction, the sum of the kinetic
... (b) Mechanical energy is E K U . From the graph, U 20 J at x 1.0 m. The kinetic energy is K 12 mv 2 12 (0.100 kg) (25 m/s) 2 31.25 J. Thus E 51.25 J. (c) The total energy line at 51.25 J is shown on the graph above. (d) The turning point occurs where the total energy line crosses the ...
... (b) Mechanical energy is E K U . From the graph, U 20 J at x 1.0 m. The kinetic energy is K 12 mv 2 12 (0.100 kg) (25 m/s) 2 31.25 J. Thus E 51.25 J. (c) The total energy line at 51.25 J is shown on the graph above. (d) The turning point occurs where the total energy line crosses the ...
Glossary of Key Terms in Chapter Two
... mass number (2.1 ) the sum of the number of protons and neutrons in an atom. metal (2.4) element located on the left side of the periodic table (left of the “staircase” boundary). metalloid (2.4) an element along the “staircase” boundary between metals and nonmetals; they exhibit both metallic and n ...
... mass number (2.1 ) the sum of the number of protons and neutrons in an atom. metal (2.4) element located on the left side of the periodic table (left of the “staircase” boundary). metalloid (2.4) an element along the “staircase” boundary between metals and nonmetals; they exhibit both metallic and n ...
Element: pure substances that are made up of one kind of atom
... Nucleus: located at the center of the atom. Consists of protons and neutrons. Electrons surround the nucleus. Electron Cloud: the current model of an atom contains an electron cloud that shows electrons traveling in specific energy levels around a nucleus. The farther away from the nucleus an electr ...
... Nucleus: located at the center of the atom. Consists of protons and neutrons. Electrons surround the nucleus. Electron Cloud: the current model of an atom contains an electron cloud that shows electrons traveling in specific energy levels around a nucleus. The farther away from the nucleus an electr ...
Chapter_5
... • For elements in the third row (third energy level), once the s and p orbitals are filled you may expect the d orbitals to begin filling. • However, because the 4s sublevel is of lower energy than the 3d sublevel, 4s fills before 3d. • Remember that the configurations are written by increasing ener ...
... • For elements in the third row (third energy level), once the s and p orbitals are filled you may expect the d orbitals to begin filling. • However, because the 4s sublevel is of lower energy than the 3d sublevel, 4s fills before 3d. • Remember that the configurations are written by increasing ener ...
hdwsmp2011 - FSU High Energy Physics
... Colliding bunches of protons and anti-protons; bunches meet each other every 396 ns in the center of two detectors (DØ and CDF) (steered apart at other places) Each particle has ~ 980 GeV of energy, so the total energy in the center of mass ...
... Colliding bunches of protons and anti-protons; bunches meet each other every 396 ns in the center of two detectors (DØ and CDF) (steered apart at other places) Each particle has ~ 980 GeV of energy, so the total energy in the center of mass ...
Nature`s Book Keeping System
... concerning this point: our universe appears to be almost flat, too flat to make sense in an uncensored Einsteinian theory. One guiding principle was strongly adhered to by practically all researchers: anything as small as molecules, atoms, subatomic particles and other energy quanta, or smaller, alw ...
... concerning this point: our universe appears to be almost flat, too flat to make sense in an uncensored Einsteinian theory. One guiding principle was strongly adhered to by practically all researchers: anything as small as molecules, atoms, subatomic particles and other energy quanta, or smaller, alw ...
Quantum Tunneling
... passing through. That means there is a chance that all the atoms of the ball line up with all the empty space in the wall and the ball passes through. So, there is a possibility that when thrown the ball will pass through the wall. That’s exactly what quantum ...
... passing through. That means there is a chance that all the atoms of the ball line up with all the empty space in the wall and the ball passes through. So, there is a possibility that when thrown the ball will pass through the wall. That’s exactly what quantum ...
Topic 13.2 Nuclear Physics
... • We can illustrate how the neutrino accounts for this discrepancy by referring to the figure below showing the energy levels of a fictitious daughter nucleus and possible decay routes of the parent nucleus undergoing β+ decay. The figure shows how the neutrino accounts for the continuous β spectrum ...
... • We can illustrate how the neutrino accounts for this discrepancy by referring to the figure below showing the energy levels of a fictitious daughter nucleus and possible decay routes of the parent nucleus undergoing β+ decay. The figure shows how the neutrino accounts for the continuous β spectrum ...
1 PHY831 - Subject Exam Dec. 14th 2011, 10am - 1pm
... FM F = −kB T lnZM F = 2JN m3 − kB T N ln[2Cosh(3βJm2 )] ...
... FM F = −kB T lnZM F = 2JN m3 − kB T N ln[2Cosh(3βJm2 )] ...
Particles and Waves Answers
... energy of which is dependent on the frequency of the photon. The photon energy can be calculated using the equation E = hf. Below a certain frequency the energy absorbed by an electron in the metal, from the photon, is below that required to escape from the metal atom, thus the current in the circui ...
... energy of which is dependent on the frequency of the photon. The photon energy can be calculated using the equation E = hf. Below a certain frequency the energy absorbed by an electron in the metal, from the photon, is below that required to escape from the metal atom, thus the current in the circui ...
Theory of electrons and positrons P A. M. D
... From general philosophical grounds one would at first sight like to have as few kinds of elementary particles as possible, say only one kind, or at most two, and to have all matter built up of these elementary kinds. It appears from the experimental results, though, that there must be more than this ...
... From general philosophical grounds one would at first sight like to have as few kinds of elementary particles as possible, say only one kind, or at most two, and to have all matter built up of these elementary kinds. It appears from the experimental results, though, that there must be more than this ...
Atoms, Molecules and Bonds
... (atomic mass unit) The atomic mass of an atom is found by adding the number of protons & neutrons in an atom ...
... (atomic mass unit) The atomic mass of an atom is found by adding the number of protons & neutrons in an atom ...
UNIT 5-Ch 11-12 studyguide 2017
... 10) The electromagnetic force in an atom keeps the ____________________________________ in orbit around the nucleus. 11)______________________________________________ have almost no mass. 12) Most of the mass of an atom is in the ______________________________________. 13) Atoms with the same number ...
... 10) The electromagnetic force in an atom keeps the ____________________________________ in orbit around the nucleus. 11)______________________________________________ have almost no mass. 12) Most of the mass of an atom is in the ______________________________________. 13) Atoms with the same number ...
Physics 9 Fall 2009 - faculty.ucmerced.edu
... The sun is powered by fusion, with four protons fusing together to form a helium nucleus (two of the protons turn into neutrons) and, in the process, releasing a large amount of thermal energy. The process happens in several steps, not all at once. In one step, two protons fuse together, with one pr ...
... The sun is powered by fusion, with four protons fusing together to form a helium nucleus (two of the protons turn into neutrons) and, in the process, releasing a large amount of thermal energy. The process happens in several steps, not all at once. In one step, two protons fuse together, with one pr ...
Chemistry TEST 4 Review and Answers
... Chapter 5 Electrons in atoms and Chapter 6.3 Periodic Trends ...
... Chapter 5 Electrons in atoms and Chapter 6.3 Periodic Trends ...