May 2006
... Consider two particles of mass m moving in one dimension. Particle 1 moves freely, while particle 2 experiences a harmonic potential V (x2 ) = 21 mω 2 x22 . The two particles interact via a delta function potential Vint (x12 ) = λδ(x12 ), with x12 ≡ x1 − x2 . Particle 2 starts in the ground state |ψ ...
... Consider two particles of mass m moving in one dimension. Particle 1 moves freely, while particle 2 experiences a harmonic potential V (x2 ) = 21 mω 2 x22 . The two particles interact via a delta function potential Vint (x12 ) = λδ(x12 ), with x12 ≡ x1 − x2 . Particle 2 starts in the ground state |ψ ...
Nuclear Magnetic Resonance Spectroscopy
... NMR Signals ¾ The number of signals shows how many different kinds of protons are present. ¾ The location of the signals shows how shielded or deshielded the proton is. ¾ The intensity of the signal shows the number of protons of that type. ¾ Signal splitting shows the number of protons ...
... NMR Signals ¾ The number of signals shows how many different kinds of protons are present. ¾ The location of the signals shows how shielded or deshielded the proton is. ¾ The intensity of the signal shows the number of protons of that type. ¾ Signal splitting shows the number of protons ...
ATOMS
... a. If an atom gains or loses electrons it is called an ion. b. Gaining or losing an electron gives the atom a charge. – The charge could be positive (if the atom loses electrons) or negative (if it gains electrons). ...
... a. If an atom gains or loses electrons it is called an ion. b. Gaining or losing an electron gives the atom a charge. – The charge could be positive (if the atom loses electrons) or negative (if it gains electrons). ...
L01_5342_Sp02
... Notes 1. This syllabus may be changed by the instructor as needed for good adademic practice. 2. Quizzes & tests: open book (no Xerox copies) OR one handwritten page of notes. Calculator OK. L1 January 15 ...
... Notes 1. This syllabus may be changed by the instructor as needed for good adademic practice. 2. Quizzes & tests: open book (no Xerox copies) OR one handwritten page of notes. Calculator OK. L1 January 15 ...
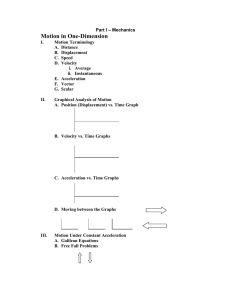
Part I – Mechanics
... i. Glow of objects at high temperatures ii. All objects emit E/M radiation. Infrared at low temp and visible light at high temp. Scientist couldn’t understand why different wavelengths at different temperatures. ...
... i. Glow of objects at high temperatures ii. All objects emit E/M radiation. Infrared at low temp and visible light at high temp. Scientist couldn’t understand why different wavelengths at different temperatures. ...
Potential Energy of a system of charges
... Initially, total energy = K.E. of He+2 (P.E. = zero since d = ∞) At closest interaction, total energy = P.E. = keQ1Q2 / d d = keQ1Q2 / K.E. K.E. = 1/2 mHev2 = 1/2 (4*1.67x10-27kg)(1x107m/s)2 = 3.3x10-13 J d = (9x109 Nm2/C2)(2)(79)(1.6x10-19C)2 / (3.3x10-13J) = 1.1x10-13 m = 110 fm Size of nucleus mu ...
... Initially, total energy = K.E. of He+2 (P.E. = zero since d = ∞) At closest interaction, total energy = P.E. = keQ1Q2 / d d = keQ1Q2 / K.E. K.E. = 1/2 mHev2 = 1/2 (4*1.67x10-27kg)(1x107m/s)2 = 3.3x10-13 J d = (9x109 Nm2/C2)(2)(79)(1.6x10-19C)2 / (3.3x10-13J) = 1.1x10-13 m = 110 fm Size of nucleus mu ...
nuclear physics - The Physics Cafe
... A Incorrect. When a nucleus with a mass number less than about 80 splits into smaller nuclei, there is a decrease in the binding energy per nucleon, hence, energy is required to trigger the fission process i.e. energy is absorbed.. B Correct. When a nucleus with a mass number greater than 80 fuses w ...
... A Incorrect. When a nucleus with a mass number less than about 80 splits into smaller nuclei, there is a decrease in the binding energy per nucleon, hence, energy is required to trigger the fission process i.e. energy is absorbed.. B Correct. When a nucleus with a mass number greater than 80 fuses w ...
Exam 2 (word)
... 5) A resistor has a potential drop of 5V when a current of 1.25A flows through it. If all other variables remain constant, what is the current through the resistor if its length is tripled? a) 0.104A b) 0.209A c) 0.417A d) 0.833A e) not enough information 6) Can a charged particle be moved through a ...
... 5) A resistor has a potential drop of 5V when a current of 1.25A flows through it. If all other variables remain constant, what is the current through the resistor if its length is tripled? a) 0.104A b) 0.209A c) 0.417A d) 0.833A e) not enough information 6) Can a charged particle be moved through a ...
The Atom
... particles should not be deflected much. This is because the positive charge in an atom is uniformly distributed within the atom. ...
... particles should not be deflected much. This is because the positive charge in an atom is uniformly distributed within the atom. ...
A POSSIBLE ENHANCEMENT MECHANISM OF NUCLEAR FUSION
... microscopic scale. They are induced not only by an externally applied field but also they naturally exist as counterstreams of free electrons. They exist inside and/or outside of bulk metals. Such electron currents make regions where the electric potential is negative. And nuclei gather there and mu ...
... microscopic scale. They are induced not only by an externally applied field but also they naturally exist as counterstreams of free electrons. They exist inside and/or outside of bulk metals. Such electron currents make regions where the electric potential is negative. And nuclei gather there and mu ...
quant13
... • For an isolated atom in vacuum, including the hyperfine n, l , s , j , f , m f interaction, general state now looks something like • Since rotating the atom changes mf different mf values must be truly degenerate • Consider a uniform magnetic field acting on an atom B Bzˆ • Ignore hyperfine spli ...
... • For an isolated atom in vacuum, including the hyperfine n, l , s , j , f , m f interaction, general state now looks something like • Since rotating the atom changes mf different mf values must be truly degenerate • Consider a uniform magnetic field acting on an atom B Bzˆ • Ignore hyperfine spli ...
CH 21
... B. Nuclear Binding Energy – Atoms are not the sum of parts, there is always missing mass which is called the binding energy. Calculate the binding energy per nucleon for 6Li. The atomic mass of 6Li is 6.01521 amu, mass of a proton is 1.00728 amu, mass of a neutron is 1.008665 amu and mass of an elec ...
... B. Nuclear Binding Energy – Atoms are not the sum of parts, there is always missing mass which is called the binding energy. Calculate the binding energy per nucleon for 6Li. The atomic mass of 6Li is 6.01521 amu, mass of a proton is 1.00728 amu, mass of a neutron is 1.008665 amu and mass of an elec ...
The (n,2p) Reaction as a Probe for the Pre
... Scintillation is the flash of light that occurs in certain organic materials as a result of molecular transitions caused by ionizing radiation passing through the scintillator. This radiation causes the scintillator’s molecules to excite from a ground state to a higher energy state. Once in this hig ...
... Scintillation is the flash of light that occurs in certain organic materials as a result of molecular transitions caused by ionizing radiation passing through the scintillator. This radiation causes the scintillator’s molecules to excite from a ground state to a higher energy state. Once in this hig ...
• Slip quiz - • Notes- Atoms - back to • Isotopes Notes (POGIL
... The Strong Nuclear Force protons are held together in the nucleus by the strong nuclear force that acts over short range (short distances) inside the nucleus only and can overcome the electrostatic repulsion between the positive protons (Note: There is accepted data from experiments to support each ...
... The Strong Nuclear Force protons are held together in the nucleus by the strong nuclear force that acts over short range (short distances) inside the nucleus only and can overcome the electrostatic repulsion between the positive protons (Note: There is accepted data from experiments to support each ...
Chapter 5 Atomic Structure and the Periodic Table Early
... Early Models of the Atom Democritus who lived in Greece in the fourth century B.C. was the first to suggest that matter is composed of tiny fundamental particles called __________________. He believed that atoms were ... There was no scientific testing at this time. John Dalton(1766-1844) An English ...
... Early Models of the Atom Democritus who lived in Greece in the fourth century B.C. was the first to suggest that matter is composed of tiny fundamental particles called __________________. He believed that atoms were ... There was no scientific testing at this time. John Dalton(1766-1844) An English ...
Name:
... nucleus. When presented with problems you need to be able to tell me how many proton neutrons and electron are in an element or isotope based on limited information, such as: If I give you the hyphen notation or nuclear symbol of an uranium isotope you should be able to tell me how many protons, neu ...
... nucleus. When presented with problems you need to be able to tell me how many proton neutrons and electron are in an element or isotope based on limited information, such as: If I give you the hyphen notation or nuclear symbol of an uranium isotope you should be able to tell me how many protons, neu ...