Worksheet 2.1 - contentextra
... radioactive atom. Alpha decay results in a decrease of the atomic number of the element by two and a decrease in the mass number by four. Alpha particle decay. ...
... radioactive atom. Alpha decay results in a decrease of the atomic number of the element by two and a decrease in the mass number by four. Alpha particle decay. ...
Practice problems - Phenix at Vanderbilt
... levels in this nucleus are not precisely the same as though in the Bohr formula since the electrons are not only affected by the Z=2 nucleus, but they are also affected by each other. However, if one of the electrons from a He atom is removed, this positively charged ion (a 42 He nucleus plus only O ...
... levels in this nucleus are not precisely the same as though in the Bohr formula since the electrons are not only affected by the Z=2 nucleus, but they are also affected by each other. However, if one of the electrons from a He atom is removed, this positively charged ion (a 42 He nucleus plus only O ...
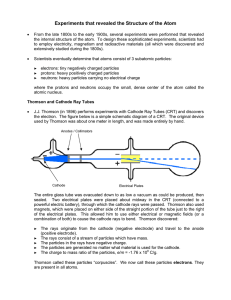
Experiments that revealed the Structure of the Atom
... From the late 1800s to the early 1900s, several experiments were performed that revealed the internal structure of the atom. To design these sophisticated experiments, scientists had to employ electricity, magnetism and radioactive materials (all which were discovered and extensively studied during ...
... From the late 1800s to the early 1900s, several experiments were performed that revealed the internal structure of the atom. To design these sophisticated experiments, scientists had to employ electricity, magnetism and radioactive materials (all which were discovered and extensively studied during ...
solutions
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
Document
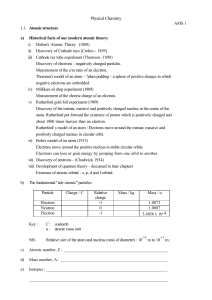
... one another but different from atoms of all other elements atoms of one element cannot be changed into another, created or destroyed compounds are formed when atoms combine in new ways Dalton’s atomic theory explained - law of constant composition – in compound, number and kinds of atoms are con ...
... one another but different from atoms of all other elements atoms of one element cannot be changed into another, created or destroyed compounds are formed when atoms combine in new ways Dalton’s atomic theory explained - law of constant composition – in compound, number and kinds of atoms are con ...
Atoms
... (“plum pudding" model). In order to test this model the following experiment was carried out by Geiger and Marsden. Incident ...
... (“plum pudding" model). In order to test this model the following experiment was carried out by Geiger and Marsden. Incident ...
Exit Slip: Atomic Structure and Nuclear Chemistry-1
... 1. Compared to the charge and mass of a proton, an electron has (1.e) A. the same charge and a smaller mass C. the same charge and the same mass B. an opposite charge and a smaller mass D. an opposite charge and the same mass 2. Which symbols represent atoms that are isotopes (11.c)? A. C-14 and N-1 ...
... 1. Compared to the charge and mass of a proton, an electron has (1.e) A. the same charge and a smaller mass C. the same charge and the same mass B. an opposite charge and a smaller mass D. an opposite charge and the same mass 2. Which symbols represent atoms that are isotopes (11.c)? A. C-14 and N-1 ...
Nuclear Chemistry 1997 D
... in part (a) is slightly less than that of the original a(234,94) Pu because of the Binding Energy in order for fission to occur, there must be a release of neutrons, forming energy. This energy is known as the “mass defect” where mass is converted into energy as shown in Einstein’s equation ...
... in part (a) is slightly less than that of the original a(234,94) Pu because of the Binding Energy in order for fission to occur, there must be a release of neutrons, forming energy. This energy is known as the “mass defect” where mass is converted into energy as shown in Einstein’s equation ...
33 Atomic Nucleus and Radioactivity Answers and Solutions for
... 21. A transmutation is the changing of an element to another element. 22. The transmutation of thorium by alpha emission produces an element with 2 less protons in the nucleus, to atomic number 88. 23. When thorium emits a beta particle, it transmutes to an element with atomic number increased by 1, ...
... 21. A transmutation is the changing of an element to another element. 22. The transmutation of thorium by alpha emission produces an element with 2 less protons in the nucleus, to atomic number 88. 23. When thorium emits a beta particle, it transmutes to an element with atomic number increased by 1, ...
N5_Ink_Ex_1_Unit_1
... b) Antimony oxide, Sb2O3, is added to reduce any bubbles that may appear during the manufacturing process. What type of bonding will be present in Antimony oxide?___________________________________________________________(1) c) In the manufacture of glass, other chemicals can be added to alter the p ...
... b) Antimony oxide, Sb2O3, is added to reduce any bubbles that may appear during the manufacturing process. What type of bonding will be present in Antimony oxide?___________________________________________________________(1) c) In the manufacture of glass, other chemicals can be added to alter the p ...
The Atomic Zoo
... Have you ever seen a muon? You can’t see electrically charged particles directly as they travel through the Diffusion Cloud Chamber, but nevertheless, they all – alpha and beta particles, protons, muons, electrons and positrons – leave a visible condensation trail behind them. The high-speed particl ...
... Have you ever seen a muon? You can’t see electrically charged particles directly as they travel through the Diffusion Cloud Chamber, but nevertheless, they all – alpha and beta particles, protons, muons, electrons and positrons – leave a visible condensation trail behind them. The high-speed particl ...
1 slide per page() - Wayne State University Physics and Astronomy
... Binding energy for heavy nuclei is about 7.2 MeV per nucleon Binding energy for intermediate nuclei is about 8.2 MeV per nucleon Therefore, the fission fragments have less mass than the nucleons in the original nuclei This decrease in mass per nucleon appears as released energy in the fissio ...
... Binding energy for heavy nuclei is about 7.2 MeV per nucleon Binding energy for intermediate nuclei is about 8.2 MeV per nucleon Therefore, the fission fragments have less mass than the nucleons in the original nuclei This decrease in mass per nucleon appears as released energy in the fissio ...
4 slides per page() - Wayne State University Physics and
... In the first atomic bomb, the energy released was equivalent to about 30 kilotons of TNT, where a ton of TNT releases an energy of 4.0 × 109 J. The amount of mass converted into energy in this event is nearest to: (a) 1 μg, (b) 1 mg, (c) 1 g, ...
... In the first atomic bomb, the energy released was equivalent to about 30 kilotons of TNT, where a ton of TNT releases an energy of 4.0 × 109 J. The amount of mass converted into energy in this event is nearest to: (a) 1 μg, (b) 1 mg, (c) 1 g, ...
Topic 7_1_Ext D__Nuclear structure and force
... The two positive charges will want to accelerate apart with an acceleration of F ...
... The two positive charges will want to accelerate apart with an acceleration of F ...
The nucleus
... e.g. m n + m p – m D = B > 0 the potential energy of the nucleus is negative m n + m p + ( – E int ) = m D so B = E int the binding energy per nucleon B/A varies with A and is in the interval 5 MeV - 10 MeV (Krane) a A>62: B/A decreases with A : fission a A<62: B/A increases with A : fusion RAF211 - ...
... e.g. m n + m p – m D = B > 0 the potential energy of the nucleus is negative m n + m p + ( – E int ) = m D so B = E int the binding energy per nucleon B/A varies with A and is in the interval 5 MeV - 10 MeV (Krane) a A>62: B/A decreases with A : fission a A<62: B/A increases with A : fusion RAF211 - ...
Phys214 Final Exam
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...