TIMELINE OF NUCLEAR PHYSICS
... 1913 Bohr constructs his first model of the atom describing the emission of light by electrons changing their circular orbits while orbiting a positive nucleus. model can actually predict wavelength of light based on equations representing his mechanical model of the atom. This was an incredible ad ...
... 1913 Bohr constructs his first model of the atom describing the emission of light by electrons changing their circular orbits while orbiting a positive nucleus. model can actually predict wavelength of light based on equations representing his mechanical model of the atom. This was an incredible ad ...
Midterm Review Sheet
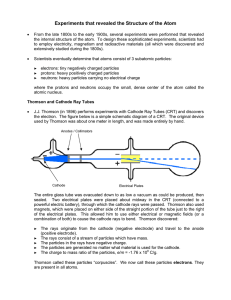
... 6. Crookes - using Crookes' tube discovered "cathode rays" as negative particles/radiation. This was the first evidence of electrons. 7. Thomson – determined: 1) that cathode rays were actually streams of negatively charged particles called electrons (could be deflected by a magnetic or electric fie ...
... 6. Crookes - using Crookes' tube discovered "cathode rays" as negative particles/radiation. This was the first evidence of electrons. 7. Thomson – determined: 1) that cathode rays were actually streams of negatively charged particles called electrons (could be deflected by a magnetic or electric fie ...
3. (a) The force on the electron is Thus, the magnitude of FB is 6.2
... (b) This amounts to repeating the above computation with a change in the sign in the charge. Thus, FB has the same magnitude but points in the negative z direction, namely, ...
... (b) This amounts to repeating the above computation with a change in the sign in the charge. Thus, FB has the same magnitude but points in the negative z direction, namely, ...
physics 30 Matter assignment 4 - ND
... An electron passes undeflected through perpendicular electric magnetic fields that have strengths of 2.4 x 103 N/C and 3.0 x 10-2 T respectively. The speed of the electron, expressed in scientific notation, is b x 10w m/s. The value of w is __________. (Write the value of w, do not use significant d ...
... An electron passes undeflected through perpendicular electric magnetic fields that have strengths of 2.4 x 103 N/C and 3.0 x 10-2 T respectively. The speed of the electron, expressed in scientific notation, is b x 10w m/s. The value of w is __________. (Write the value of w, do not use significant d ...
cyclotron
... particle enter into other chamber with grater velocity.it performs greater semicircular path in the chamber. This process continues till the particle is ejected out with a strong deflection. ...
... particle enter into other chamber with grater velocity.it performs greater semicircular path in the chamber. This process continues till the particle is ejected out with a strong deflection. ...
Deflection of Beta Particles in Magnetic Field
... change the direction of charged particles and follow a circular path at constant velocity in the magnetic field. So that the magnetic field cause Beta particles to change direction as the particles cross this field. ...
... change the direction of charged particles and follow a circular path at constant velocity in the magnetic field. So that the magnetic field cause Beta particles to change direction as the particles cross this field. ...
Flashback 2
... rose to 56 cm above the initial position, Joseph’s velocity was 4.0 m/s. His mass is 45 kg. ...
... rose to 56 cm above the initial position, Joseph’s velocity was 4.0 m/s. His mass is 45 kg. ...
The Atomic Zoo
... The higher the atomic number of a chemical element, the better it is at absorbing X-rays. Calcium (Z=20) is better than Hydrogen (Z=1) and Carbon (Z=6). This is how X-ray pictures distinguish bones from soft tissues. Besides absorption via the photo-electric effect, there is another (but much smalle ...
... The higher the atomic number of a chemical element, the better it is at absorbing X-rays. Calcium (Z=20) is better than Hydrogen (Z=1) and Carbon (Z=6). This is how X-ray pictures distinguish bones from soft tissues. Besides absorption via the photo-electric effect, there is another (but much smalle ...
Goal 4.01
... Atoms of the same element have the same mass and other properties. Atoms cannot be created or destroyed. Compounds contain the atoms of more than one element. In chemical reactions, atoms of the same element always combine, separate, or rearrange in the same way. ...
... Atoms of the same element have the same mass and other properties. Atoms cannot be created or destroyed. Compounds contain the atoms of more than one element. In chemical reactions, atoms of the same element always combine, separate, or rearrange in the same way. ...
HW4 - SMU Physics
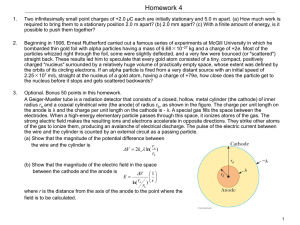
... Optional. Bonus 50 points in this homework. A Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius rb, as shown in the figure. The charge per unit length on the anode is λ a ...
... Optional. Bonus 50 points in this homework. A Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius rb, as shown in the figure. The charge per unit length on the anode is λ a ...
History of subatomic physics
.jpg?width=300)
The idea that matter consists of smaller particles and that there exists a limited number of sorts of primary, smallest particles in nature has existed in natural philosophy since time immemorial. Such ideas gained physical credibility beginning in the 19th century, but the concept of ""elementary particle"" underwent some changes in its meaning: notably, modern physics no longer deems elementary particles indestructible. Even elementary particles can decay or collide destructively; they can cease to exist and create (other) particles in result.Increasingly small particles have been discovered and researched: they include molecules, which are constructed of atoms, that in turn consist of subatomic particles, namely atomic nuclei and electrons. Many more types of subatomic particles have been found. Most such particles (but not electrons) were eventually found to be composed of even smaller particles such as quarks. Particle physics studies these smallest particles and their behaviour under high energies, whereas nuclear physics studies atomic nuclei and their (immediate) constituents: protons and neutrons.