Stoichiometry - hrsbstaff.ednet.ns.ca
... define aromatics as compounds similar to benzene in their bonding draw electron dot diagrams to show the bonding in ethane, ethene, and ethyne describing the bonding in benzene using the term "delocalized electrons", and explaining how its unreactive nature and the equal C-C bond lengths in benzene ...
... define aromatics as compounds similar to benzene in their bonding draw electron dot diagrams to show the bonding in ethane, ethene, and ethyne describing the bonding in benzene using the term "delocalized electrons", and explaining how its unreactive nature and the equal C-C bond lengths in benzene ...
Molecular Orbitals and Hybridisation
... structure, benzene is a very stable, saturated structure that does not undergo addition reactions. ...
... structure, benzene is a very stable, saturated structure that does not undergo addition reactions. ...
Organic chemistry: introduction
... When you are drawing the three stages of a mechanism, you must think of and comment on different things. Stage 1: Here the most important thing to think about is getting the two particles lined up properly so that the oppositely charged bits are next to each other. In your labeling, you should try t ...
... When you are drawing the three stages of a mechanism, you must think of and comment on different things. Stage 1: Here the most important thing to think about is getting the two particles lined up properly so that the oppositely charged bits are next to each other. In your labeling, you should try t ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... 29) What results from an unequal sharing of electrons between atoms? A) a nonpolar covalent bond B) a polar covalent bond C) an ionic bond D) a hydrophobic interaction 30) A covalent bond is likely to be polar when _____. A) one of the atoms sharing electrons is more electronegative than the other ...
... 29) What results from an unequal sharing of electrons between atoms? A) a nonpolar covalent bond B) a polar covalent bond C) an ionic bond D) a hydrophobic interaction 30) A covalent bond is likely to be polar when _____. A) one of the atoms sharing electrons is more electronegative than the other ...
Aromatic amines The
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
CHEM1405 2005-J-3 June 2005 • Ammonia (NH 3) has a boiling
... • Explain, in terms of chemical bonding and intermolecular forces, the following trend in melting points: CH4 < I2 < NaCl < silica (SiO2) There are only dispersion forces between the molecules in CH4 and I2. The I atom is a large, many-electron atom so its electron cloud is more easily polarised tha ...
... • Explain, in terms of chemical bonding and intermolecular forces, the following trend in melting points: CH4 < I2 < NaCl < silica (SiO2) There are only dispersion forces between the molecules in CH4 and I2. The I atom is a large, many-electron atom so its electron cloud is more easily polarised tha ...
What is Organic Chemistry? - Westgate Mennonite Collegiate
... structure and activity of life. They are building materials for living cells, appearing in the structures inside the cell and within the cell membrane. While many of the proteins are structural proteins, many are regulatory proteins called enzymes. They contain carbon, hydrogen, and oxygen like the ...
... structure and activity of life. They are building materials for living cells, appearing in the structures inside the cell and within the cell membrane. While many of the proteins are structural proteins, many are regulatory proteins called enzymes. They contain carbon, hydrogen, and oxygen like the ...
Chapter 1 Glossary The Nature of Chemistry
... The intermolecular attraction between a nitrogen, oxygen, or fluorine atom of one molecule and a hydrogen atom bonded to a nitrogen, oxygen, or fluorine atom in another molecule. Metallic bond The attraction between the positive metal cations that form the basic structure of a solid metal and the ne ...
... The intermolecular attraction between a nitrogen, oxygen, or fluorine atom of one molecule and a hydrogen atom bonded to a nitrogen, oxygen, or fluorine atom in another molecule. Metallic bond The attraction between the positive metal cations that form the basic structure of a solid metal and the ne ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16 a. Explain, with theory the spectrum of a linear diatomic molecule of rigid rotor type. Outline the correction for non-rigid type. (10.5) 16 b. The rotational constant of NO is 1.7201 cm-1. Calculate the moment of inertia of the molecule about a line perpendicular to its axis. (2) 17 a. Outline b ...
... 16 a. Explain, with theory the spectrum of a linear diatomic molecule of rigid rotor type. Outline the correction for non-rigid type. (10.5) 16 b. The rotational constant of NO is 1.7201 cm-1. Calculate the moment of inertia of the molecule about a line perpendicular to its axis. (2) 17 a. Outline b ...
AP_Summer_Naming
... Write the formula and name of the ionic compound that would result from the combination of the following ions ...
... Write the formula and name of the ionic compound that would result from the combination of the following ions ...
Chapter 8 Notes
... And for negative ions: Chlorine Sulfur Phosphorus Try p. 214 # 2 – 5 Formation of Ionic Compounds: Now that we have an idea how ions are formed we can explain how ionic compounds form. We will use Sodium Chloride as a first example. The sodium atom looses an electron to become positively charged. Th ...
... And for negative ions: Chlorine Sulfur Phosphorus Try p. 214 # 2 – 5 Formation of Ionic Compounds: Now that we have an idea how ions are formed we can explain how ionic compounds form. We will use Sodium Chloride as a first example. The sodium atom looses an electron to become positively charged. Th ...
Slide 1
... 3) p-MO’s of cyclic conjugated ligands For qualitative analysis of bonding between transition metals and p-electron donors such as organic compounds with C=C bonds it is necessary to know how the ligand p-MO’s look like. Frost diagrams allow establish the shape, degeneracy and the energy sequence o ...
... 3) p-MO’s of cyclic conjugated ligands For qualitative analysis of bonding between transition metals and p-electron donors such as organic compounds with C=C bonds it is necessary to know how the ligand p-MO’s look like. Frost diagrams allow establish the shape, degeneracy and the energy sequence o ...
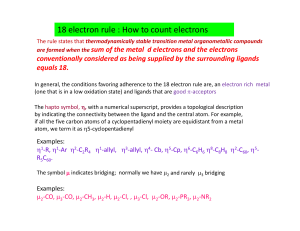
18 electron rule : How to count electrons
... In general, the conditions favoring adherence to the 18 electron rule are, an electron rich metal (one that is in a low oxidation state) and ligands that are good π‐acceptors The hapto symbol, η, with a numerical superscript, provides a topological description by indicating the connectivity betw ...
... In general, the conditions favoring adherence to the 18 electron rule are, an electron rich metal (one that is in a low oxidation state) and ligands that are good π‐acceptors The hapto symbol, η, with a numerical superscript, provides a topological description by indicating the connectivity betw ...
04_Lecture_Presentation
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
Chapter 2 The Chemical Context of Life About 25 of the 92 natural
... 6. From its atomic number of 15, it is possible to predict that the phosphorus atom has A) 15 neutrons. B) 15 protons. C) 15 electrons. D) 8 electrons in its outermost electron shell. E) 15 protons and 15 electrons. Answer: E Topic: Concept 2.2 7. A covalent chemical bond is one in which A) electro ...
... 6. From its atomic number of 15, it is possible to predict that the phosphorus atom has A) 15 neutrons. B) 15 protons. C) 15 electrons. D) 8 electrons in its outermost electron shell. E) 15 protons and 15 electrons. Answer: E Topic: Concept 2.2 7. A covalent chemical bond is one in which A) electro ...
FUNCTIONAL GROUPS
... • C-N, N-H, C=O bonds are polar, so molecules are usually polar • Primary and secondary amides experience hydrogen bonding • Soluble in water and other polar solvents, solubility decreases as the number of carbons increases • Primary amides have higher melting and boiling points than analogous ...
... • C-N, N-H, C=O bonds are polar, so molecules are usually polar • Primary and secondary amides experience hydrogen bonding • Soluble in water and other polar solvents, solubility decreases as the number of carbons increases • Primary amides have higher melting and boiling points than analogous ...
Organic Chemistry Syllabus and Course Outline
... nomenclature, chemical and physical properties, structures, mechanisms, common molecules, and the diversity of organic molecules in plants, bacteria, and animals. Many chapters in our textbook also integrate the societal, pharmaceutical or industrial importance of specific compounds. An important as ...
... nomenclature, chemical and physical properties, structures, mechanisms, common molecules, and the diversity of organic molecules in plants, bacteria, and animals. Many chapters in our textbook also integrate the societal, pharmaceutical or industrial importance of specific compounds. An important as ...
Sample Exam 1
... 10. (6 pts) Rank the following analgesics in order of increasing water solubility (1 – least soluble, 4 – most soluble): ...
... 10. (6 pts) Rank the following analgesics in order of increasing water solubility (1 – least soluble, 4 – most soluble): ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.