Alkynes
... • Compounds with two triple bonds are named as diynes, those with three are named as triynes and so forth. • Compounds both a double and triple bond are named as enynes. The chain is numbered to give the first site of unsaturation (either C=C or C≡C) the lower number. • The simplest alkyne, H-C≡C-H, ...
... • Compounds with two triple bonds are named as diynes, those with three are named as triynes and so forth. • Compounds both a double and triple bond are named as enynes. The chain is numbered to give the first site of unsaturation (either C=C or C≡C) the lower number. • The simplest alkyne, H-C≡C-H, ...
chapter 9 - chemical bonds
... octet. Examples are: PCl5, SF4, SF6, BrF5, XeF2, and XeF4. They involve species containing elements of the third period or higher which have empty (n-1)d orbitals in their valence shell. ...
... octet. Examples are: PCl5, SF4, SF6, BrF5, XeF2, and XeF4. They involve species containing elements of the third period or higher which have empty (n-1)d orbitals in their valence shell. ...
PowerPoint **
... Draw mechanisms for the following reaction and explain why carbonyl acid can’t undergo similar reaction ...
... Draw mechanisms for the following reaction and explain why carbonyl acid can’t undergo similar reaction ...
KS4 Organic Chemistry – Alkanes
... Carbon is an unusual atom in that it is able to form very strong covalent bonds with other carbon atoms. When we then include it’s ability to also bond with other elements we open up the possibility of the highly diverse and complex molecules (like DNA) that have led to the possibility of life. Beca ...
... Carbon is an unusual atom in that it is able to form very strong covalent bonds with other carbon atoms. When we then include it’s ability to also bond with other elements we open up the possibility of the highly diverse and complex molecules (like DNA) that have led to the possibility of life. Beca ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... 6. if counting from either end is tie in yne #, use subst # for tie-breaker (look for 1st point of difference) 7. if still tied, go to second subst, etc (1st point of difference) 8. #1 in cyclics, other C in pi bond is #2 a. no need to include number of pi bond in name unless diene, when ...
... 6. if counting from either end is tie in yne #, use subst # for tie-breaker (look for 1st point of difference) 7. if still tied, go to second subst, etc (1st point of difference) 8. #1 in cyclics, other C in pi bond is #2 a. no need to include number of pi bond in name unless diene, when ...
Document
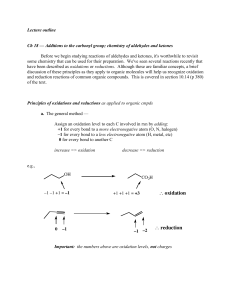
... C—Z bonds (usually C—O bonds) or a decrease in the number of C—H bonds. • Recall that reduction results in a decrease in the number of C—Z bonds (usually C—O bonds) or an increase in the number of C—H bonds. Figure 12.1 A general scheme for the oxidation and reduction of a carbon compound ...
... C—Z bonds (usually C—O bonds) or a decrease in the number of C—H bonds. • Recall that reduction results in a decrease in the number of C—Z bonds (usually C—O bonds) or an increase in the number of C—H bonds. Figure 12.1 A general scheme for the oxidation and reduction of a carbon compound ...
Fundamentals Of Organic Chemistry
... that instead of the expected neopentyl alcohol (Me3CCH2OH), due to alkyl shift, 2-methylbutan-2-ol and 2-methyl but-2-ene are formed. ...
... that instead of the expected neopentyl alcohol (Me3CCH2OH), due to alkyl shift, 2-methylbutan-2-ol and 2-methyl but-2-ene are formed. ...
Document
... e) Different in hardness between anhydrous and hydrated salt Anhydrous magnesium sulphate solid is much ___________ than its hydrated form (MgSO4.7H2O), this is because in anhydrous form, the cations and anions are linked by _________________________; while in the hydrated form, water molecules are ...
... e) Different in hardness between anhydrous and hydrated salt Anhydrous magnesium sulphate solid is much ___________ than its hydrated form (MgSO4.7H2O), this is because in anhydrous form, the cations and anions are linked by _________________________; while in the hydrated form, water molecules are ...
Double Bond
... Thermal isomerization allows us to measure the strength of the pi bond. Thermal isomerization involves the interconversion of the cis form and the trans form of a double bond at high temperature. During the isomerization process, the bond between the two carbon atoms is broken and the p orbitals ...
... Thermal isomerization allows us to measure the strength of the pi bond. Thermal isomerization involves the interconversion of the cis form and the trans form of a double bond at high temperature. During the isomerization process, the bond between the two carbon atoms is broken and the p orbitals ...
LESSON-3
... D. highly electropositive oxygen atoms. E. highly electropositive nitrogen atoms. 39. This functional group is weakly basic because it can accept an H+ ion: A. hydroxyl B. carbonyl C. amino D. phosphate E. sulfhydryl 40. Hydrocarbons are hydrophobic because: A. the covalent bonds between hydrogen an ...
... D. highly electropositive oxygen atoms. E. highly electropositive nitrogen atoms. 39. This functional group is weakly basic because it can accept an H+ ion: A. hydroxyl B. carbonyl C. amino D. phosphate E. sulfhydryl 40. Hydrocarbons are hydrophobic because: A. the covalent bonds between hydrogen an ...
Topic 20 IB Chemistry Definitions
... Chemistry: Definitions Topic 20 – Organic Chemistry Chiral center: ...
... Chemistry: Definitions Topic 20 – Organic Chemistry Chiral center: ...
Positive Ions: Loss of Electrons
... Groups 5A (15), 6A (16), and 7A (17) is high. Rather than lose electrons to form ions, a nonmetal atom will gain one or more electrons to obtain a stable electron configuration. ...
... Groups 5A (15), 6A (16), and 7A (17) is high. Rather than lose electrons to form ions, a nonmetal atom will gain one or more electrons to obtain a stable electron configuration. ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.