2202 Chapter 9 10 11 Partial
... The chemical formula for benzene, C6H6 , was determined by Michael Faraday in 1825. Structural formula was determined by August Kekulé in 1865. ...
... The chemical formula for benzene, C6H6 , was determined by Michael Faraday in 1825. Structural formula was determined by August Kekulé in 1865. ...
Notes
... carbon atom is bonded to another carbon and two oxygen atoms, one through a single bond and one through a double bond. That sounds pretty confusing, but esters are easy to recognize. They all have the same basic shape as the picture above, where the letter “R” means carbon atoms or other “organic” g ...
... carbon atom is bonded to another carbon and two oxygen atoms, one through a single bond and one through a double bond. That sounds pretty confusing, but esters are easy to recognize. They all have the same basic shape as the picture above, where the letter “R” means carbon atoms or other “organic” g ...
Carbon Compounds
... Alkynes • Hydrocarbons which have at least one triple bond between two carbons. We will always use one triple bond only. • Unsaturated • The naming prefixes are the same as for alkanes with an yne ending • The general formula is CnH2n-2 • pharmaceuticals ...
... Alkynes • Hydrocarbons which have at least one triple bond between two carbons. We will always use one triple bond only. • Unsaturated • The naming prefixes are the same as for alkanes with an yne ending • The general formula is CnH2n-2 • pharmaceuticals ...
PPT CH 11
... Benzene Structure – Modern • Modern concept of benzene structure is based on overlapping orbitals – Each carbon is bonded to two others by sharing a pair of electrons – These same carbon atoms also each share a pair of electrons with a hydrogen atom – Remaining 6 electrons are located in p orbitals ...
... Benzene Structure – Modern • Modern concept of benzene structure is based on overlapping orbitals – Each carbon is bonded to two others by sharing a pair of electrons – These same carbon atoms also each share a pair of electrons with a hydrogen atom – Remaining 6 electrons are located in p orbitals ...
Molecules of Life ppt
... charge (ex oxygen) and a hydrogen atom Cohesion – tendency of the molecules of a substance to stick together Adhesion – attractive force between unlike substances Capillary action – ability to spread through narrow pores or tubes against gravity Water is less dense at lower temperatures ...
... charge (ex oxygen) and a hydrogen atom Cohesion – tendency of the molecules of a substance to stick together Adhesion – attractive force between unlike substances Capillary action – ability to spread through narrow pores or tubes against gravity Water is less dense at lower temperatures ...
Ex: -F, -Cl, -Br
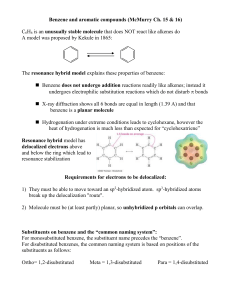
... The resonance hybrid model explains these properties of benzene: Benzene does not undergo addition reactions readily like alkenes; instead it undergoes electrophilic substitution reactions which do not disturb bonds X-ray diffraction shows all 6 bonds are equal in length (1.39 A) and that benz ...
... The resonance hybrid model explains these properties of benzene: Benzene does not undergo addition reactions readily like alkenes; instead it undergoes electrophilic substitution reactions which do not disturb bonds X-ray diffraction shows all 6 bonds are equal in length (1.39 A) and that benz ...
Section 1 Atoms, Elements, and Compounds
... o Valence- Outermost, used in chemical bonds What makes an atom neutral? Elements What is an element and what are some common elements? Atomic Number- Used to identify elements, describes number of protons. Mass Number- Mass # = Protons + Neutrons Isotopes- same number of protons, different ...
... o Valence- Outermost, used in chemical bonds What makes an atom neutral? Elements What is an element and what are some common elements? Atomic Number- Used to identify elements, describes number of protons. Mass Number- Mass # = Protons + Neutrons Isotopes- same number of protons, different ...
World of Carbon Flashcards
... on heating. They consist of polymer Thermosetting plastics do not soften or chains which have only weak forces melt on heating on account of a highly (typically van der Waals’) between cross-linked structure. them. Triglycerides are molecules formed through the condensation of one glycerol molecule ...
... on heating. They consist of polymer Thermosetting plastics do not soften or chains which have only weak forces melt on heating on account of a highly (typically van der Waals’) between cross-linked structure. them. Triglycerides are molecules formed through the condensation of one glycerol molecule ...
Chemistry Lesson 40 Organic Chemistry
... g. Some compounds have more than one functional group – such as amino acids, which contain an amine (C-N) group and an organic acid (COOH) group. h. Naming organic compounds with functional groups involves using the alkane name, and adding the prefix/suffix for the functional group. ...
... g. Some compounds have more than one functional group – such as amino acids, which contain an amine (C-N) group and an organic acid (COOH) group. h. Naming organic compounds with functional groups involves using the alkane name, and adding the prefix/suffix for the functional group. ...
Organic Nomenclature - Alkanes, Alkenes, Alkynes
... An aldehyde is an organic molecule that has an oxygen atom doubly bonded to the terminal carbon of the backbone/parent carbon chain. An aldehyde is named with the -al ending. Since the CHO must be on the terminal #1 carbon atom, the position of the CHO is not specified in the name. Example 2: Name t ...
... An aldehyde is an organic molecule that has an oxygen atom doubly bonded to the terminal carbon of the backbone/parent carbon chain. An aldehyde is named with the -al ending. Since the CHO must be on the terminal #1 carbon atom, the position of the CHO is not specified in the name. Example 2: Name t ...
Organic and Biological Molecules
... There is a separate class of cyclic unsaturated hydrocarbons called aromatic hydrocarbons. These compounds have a planar ring structure and a delocalized π system. The extended pi bonding provides exceptional stability to these molecules. Unlike other hydrocarbons, they do not burn well or cleanly. ...
... There is a separate class of cyclic unsaturated hydrocarbons called aromatic hydrocarbons. These compounds have a planar ring structure and a delocalized π system. The extended pi bonding provides exceptional stability to these molecules. Unlike other hydrocarbons, they do not burn well or cleanly. ...
Biochemistry Quiz Review
... C. sharing an electron pair. A. transferring electrons. B. transferring protons. D. sharing a proton pair. 14. In a water molecule, shared electrons spend more time around the oxygen atom than the hydrogen atoms. As a result, the oxygen atom is A. slightly positive. B. very negative. C. very positiv ...
... C. sharing an electron pair. A. transferring electrons. B. transferring protons. D. sharing a proton pair. 14. In a water molecule, shared electrons spend more time around the oxygen atom than the hydrogen atoms. As a result, the oxygen atom is A. slightly positive. B. very negative. C. very positiv ...
Study Guide for Exam 2 Chapter 12
... with more than one substituent on the benzene ring, and those in which the benzene ring is regarded as a substituent (phenyl ) group. From their names, draw structural formulas of aromatic compounds including types of compounds listed in the preceding item. Know the names and structures for the foll ...
... with more than one substituent on the benzene ring, and those in which the benzene ring is regarded as a substituent (phenyl ) group. From their names, draw structural formulas of aromatic compounds including types of compounds listed in the preceding item. Know the names and structures for the foll ...
Carbon and the Molecular Diversity of Life
... Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Single covalent bonds form a tetrahedron li ...
... Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Single covalent bonds form a tetrahedron li ...
Your Instructor
... Objectives: After the completion of this chapter you should be able to - a) name hydrocarbons (alkanes, alkenes, alkynes, aromatics); b) name alcohols, phenols, ethers, and amines; c) name acids, aldehydes, ketones, and ethers; d) identify alkanes, alkenes, alkynes and aromatics from structural form ...
... Objectives: After the completion of this chapter you should be able to - a) name hydrocarbons (alkanes, alkenes, alkynes, aromatics); b) name alcohols, phenols, ethers, and amines; c) name acids, aldehydes, ketones, and ethers; d) identify alkanes, alkenes, alkynes and aromatics from structural form ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.