Nuclear Chemistry powerpoint
... neutrons (increases the reaction rate) and extra neutrons ( creases the reaction rate). ...
... neutrons (increases the reaction rate) and extra neutrons ( creases the reaction rate). ...
The Nature of Chemical Reactions
... Decomposition Reaction: one substance breaks down, or decomposes, into two or more simpler ...
... Decomposition Reaction: one substance breaks down, or decomposes, into two or more simpler ...
Unit 13 Worksheet Answers
... 1) What is meant by the term "rate of reaction"? How fast a reaction occurs 2) It is found that a 10oC increase in temperature roughly doubles the rate of many chemical reactions. If a reaction takes 20 seconds at 40oC, how long would it take at 60oC? 5 seconds 3) Use the collision theory to explain ...
... 1) What is meant by the term "rate of reaction"? How fast a reaction occurs 2) It is found that a 10oC increase in temperature roughly doubles the rate of many chemical reactions. If a reaction takes 20 seconds at 40oC, how long would it take at 60oC? 5 seconds 3) Use the collision theory to explain ...
Atomic Nuclei - RAJEEV Classes
... The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton. ...
... The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton. ...
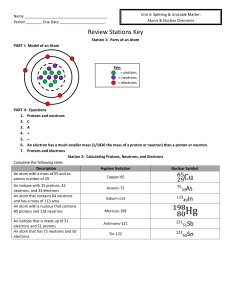
Name Period ______ Due Date Review Stations Key Station 1
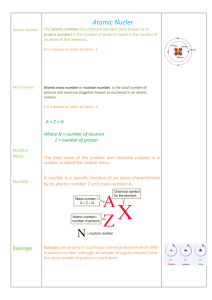
... 6. An electron has a much smaller mass (1/1836 the mass of a proton or neutron) than a proton or neutron. 7. Protons and electrons Station 2: Calculating Protons, Neutrons, and Electrons Complete the following table. Description Hyphen Notation Nuclear Symbol An atom with a mass of 65 and an Copper- ...
... 6. An electron has a much smaller mass (1/1836 the mass of a proton or neutron) than a proton or neutron. 7. Protons and electrons Station 2: Calculating Protons, Neutrons, and Electrons Complete the following table. Description Hyphen Notation Nuclear Symbol An atom with a mass of 65 and an Copper- ...
Topics Convocatory 8th Topics Objectives Sources States of Matter
... Determine the atomic number and mass number of an atom. Identify the atomic number, chemical symbol, name, and average atomic mass of an element on the Periodic Table. Compare and contrast the properties of metals, nonmetals and metalloids. Describe the arrangement of elements in groups and periods ...
... Determine the atomic number and mass number of an atom. Identify the atomic number, chemical symbol, name, and average atomic mass of an element on the Periodic Table. Compare and contrast the properties of metals, nonmetals and metalloids. Describe the arrangement of elements in groups and periods ...
Chapter #20 Nuclear Chemistry
... less stable to a more stable state In nuclear reactions more stable nuclei can be achieved by combining nuclei (fusion) or splitting a nucleus (fission) Lighter elements typically undergo fusion, while elements heavier than iron undergo fission. ...
... less stable to a more stable state In nuclear reactions more stable nuclei can be achieved by combining nuclei (fusion) or splitting a nucleus (fission) Lighter elements typically undergo fusion, while elements heavier than iron undergo fission. ...
CH_8_nucleus_new
... disappear for a chain reaction to occur in a solid lump of natural U. Slow neutrons are more likely to induce fission in 235U than fast ones. To get around this problem of fast moving neutrons, they are slowed down to induce fission in 235U. ...
... disappear for a chain reaction to occur in a solid lump of natural U. Slow neutrons are more likely to induce fission in 235U than fast ones. To get around this problem of fast moving neutrons, they are slowed down to induce fission in 235U. ...
Additional Problems - AppServ Open Project 2.4.9
... temperature of about 6 × 108 K to overcome the strong Coulomb repulsion between carbon nuclei. (a) Estimate the repulsive energy barrier to fusion, using the temperature required for carbon fusion. (In other words, what is the average kinetic energy of a carbon nucleus at 6 × 108 K?) (b) Calculate t ...
... temperature of about 6 × 108 K to overcome the strong Coulomb repulsion between carbon nuclei. (a) Estimate the repulsive energy barrier to fusion, using the temperature required for carbon fusion. (In other words, what is the average kinetic energy of a carbon nucleus at 6 × 108 K?) (b) Calculate t ...
Document
... have the same kinetic energy. (a). Conservation of momentum requires the momenta of the two fragments be equal in magnitude and oppositely directed. Thus, from KE = p2/2m, the lighter alpha particle has more kinetic energy that the more massive daughter nucleus. ...
... have the same kinetic energy. (a). Conservation of momentum requires the momenta of the two fragments be equal in magnitude and oppositely directed. Thus, from KE = p2/2m, the lighter alpha particle has more kinetic energy that the more massive daughter nucleus. ...
Period 10 Activity Solutions: Nuclear Reactions
... c) The leftmost point of the graph is deuterium ( 21 H ). What is the binding energy per nucleon of deuterium? _1 MeV_ d) The next point to the right is helium ( 42 He ). What is the binding energy per nucleon of helium? _7 MeV_ e) How much energy is given off per nucleon if two deuterium nuclei fus ...
... c) The leftmost point of the graph is deuterium ( 21 H ). What is the binding energy per nucleon of deuterium? _1 MeV_ d) The next point to the right is helium ( 42 He ). What is the binding energy per nucleon of helium? _7 MeV_ e) How much energy is given off per nucleon if two deuterium nuclei fus ...
1. Look at the drawing given in the figure which has been drawn
... fusion reactor, a gas fo heavy hydrogen is fully ionized into deuteron nuclei and electrons. This collision of nuclei and electrons is known as plasma. The nuclei move randomly in the reactor core and occasionally come close enough for nuclear fusion to take place. Usually, the temperatures in the r ...
... fusion reactor, a gas fo heavy hydrogen is fully ionized into deuteron nuclei and electrons. This collision of nuclei and electrons is known as plasma. The nuclei move randomly in the reactor core and occasionally come close enough for nuclear fusion to take place. Usually, the temperatures in the r ...
Nuclear Chemistry I: Radioactivity Reading: Moore chapter 20
... particles by unstable atomic nuclei. It may refer to the energy or particles emitted. Note: The basic building blocks of the nucleus, neutrons and protons, are referred to as nucleons; forms of an element with the same atomic number and different mass numbers are isotopes. Some isotopes are stable; ...
... particles by unstable atomic nuclei. It may refer to the energy or particles emitted. Note: The basic building blocks of the nucleus, neutrons and protons, are referred to as nucleons; forms of an element with the same atomic number and different mass numbers are isotopes. Some isotopes are stable; ...
File
... • Chemical reactions may give out heat – exothermic reactions. • Chemical reactions may take in heat energy from their surroundings – endothermic reactions ...
... • Chemical reactions may give out heat – exothermic reactions. • Chemical reactions may take in heat energy from their surroundings – endothermic reactions ...
Quantum Physics and Nuclear Physics
... When a positive charged particle approaches a nucleus such as in scattering experiments (like Rutherford’s alpha particle scattering experiment) it is repelled by electrostatic forces. The distance of closest approach (d) is taken as being a measure of the radius of the nucleus (it has the same orde ...
... When a positive charged particle approaches a nucleus such as in scattering experiments (like Rutherford’s alpha particle scattering experiment) it is repelled by electrostatic forces. The distance of closest approach (d) is taken as being a measure of the radius of the nucleus (it has the same orde ...
THERMOCHEMISTRY ENERGETICS/ENTHALPY
... All reactions require energy to break bonds in the reactants, and all reactions give off energy when new bonds are made to form the products. The difference in the energy required to break the bonds and to make the new bonds can tell you whether the reaction is endothermic or exothermic. It is possi ...
... All reactions require energy to break bonds in the reactants, and all reactions give off energy when new bonds are made to form the products. The difference in the energy required to break the bonds and to make the new bonds can tell you whether the reaction is endothermic or exothermic. It is possi ...
File
... • Radioactivity - the process in which an unstable atomic nucleus emits charged particles and/or energy (also called nuclear ...
... • Radioactivity - the process in which an unstable atomic nucleus emits charged particles and/or energy (also called nuclear ...
chap7_nucleus
... disappear for a chain reaction to occur in a solid lump of natural U. Slow neutrons are more likely to induce fission in 235U than fast ones. To get around this problem of fast moving neutrons, they are slowed down to induce fission in 235U. ...
... disappear for a chain reaction to occur in a solid lump of natural U. Slow neutrons are more likely to induce fission in 235U than fast ones. To get around this problem of fast moving neutrons, they are slowed down to induce fission in 235U. ...
Science, Systems, Matter, and Energy
... Nuclear Changes Nuclear Fusion • Fusion—joining of nuclei – Isotopes of light elements are forced together at high T’s until they fuse into a heavier nucleus • Harder to accomplish than fission, but releases more energy • Fusion of H nuclei to form He nuclei is a source of energy for sun and stars ...
... Nuclear Changes Nuclear Fusion • Fusion—joining of nuclei – Isotopes of light elements are forced together at high T’s until they fuse into a heavier nucleus • Harder to accomplish than fission, but releases more energy • Fusion of H nuclei to form He nuclei is a source of energy for sun and stars ...
Radioactivity
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
Radioactivity
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
Nuclear fusion

In nuclear physics, nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new nucleus. During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy). Fusion is the process that powers active or ""main sequence"" stars.The fusion of two nuclei with lower masses than Iron-56 (which, along with Nickel-62, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements. There are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernova.Following the discovery of quantum tunneling by Friedrich Hund, in 1929 Robert Atkinson and Fritz Houtermans used the measured masses of light elements to predict that large amounts of energy could be released by fusing small nuclei. Building upon the nuclear transmutation experiments by Ernest Rutherford, carried out several years earlier, the laboratory fusion of hydrogen isotopes was first accomplished by Mark Oliphant in 1932. During the remainder of that decade the steps of the main cycle of nuclear fusion in stars were worked out by Hans Bethe. Research into fusion for military purposes began in the early 1940s as part of the Manhattan Project. Fusion was accomplished in 1951 with the Greenhouse Item nuclear test. Nuclear fusion on a large scale in an explosion was first carried out on November 1, 1952, in the Ivy Mike hydrogen bomb test.Research into developing controlled thermonuclear fusion for civil purposes also began in earnest in the 1950s, and it continues to this day. The present article is about the theory of fusion. For details of the quest for controlled fusion and its history, see the article Fusion power.