Sample pages 1 PDF
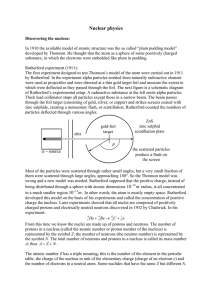
... angular distribution of the scattered projectiles is still of great importance in nuclear and particle physics, and we will encounter it repeatedly in the following chapters. These experiments established the existence of the atom as a positively charged, small, massive nucleus with negatively charg ...
... angular distribution of the scattered projectiles is still of great importance in nuclear and particle physics, and we will encounter it repeatedly in the following chapters. These experiments established the existence of the atom as a positively charged, small, massive nucleus with negatively charg ...
Nuclear Structure, Nuclear Force
... particles of approximately proton mass. In this case their energies need not exceed 5.7 MeV since in head on collision between particles of the same mass all energy is transferred to target particles – protons. ...
... particles of approximately proton mass. In this case their energies need not exceed 5.7 MeV since in head on collision between particles of the same mass all energy is transferred to target particles – protons. ...
TIMELINE OF NUCLEAR PHYSICS
... 1913 Soddy and Richards discover the property of “atomic weight” and coin term “isotope” to describe atoms of the same element that have different mass. In addition, they discover that some elements have radioactive “isotopes”, atoms of an element with different masses. They recognized that the term ...
... 1913 Soddy and Richards discover the property of “atomic weight” and coin term “isotope” to describe atoms of the same element that have different mass. In addition, they discover that some elements have radioactive “isotopes”, atoms of an element with different masses. They recognized that the term ...
Week 1: Nuclear timeline (pdf, 233 KB)
... The reader must understand that this is not a total outline of the development of physics but what I deem is the line of discoveries that leads directly to the development of atomic energy. As an example I offer thermodynamics. For this, I could develop a separate trail with some similar names such ...
... The reader must understand that this is not a total outline of the development of physics but what I deem is the line of discoveries that leads directly to the development of atomic energy. As an example I offer thermodynamics. For this, I could develop a separate trail with some similar names such ...
Nuclear models: The liquid drop model Fermi
... This average kinetic energy has a minimum at N = Z for fixed mass number A (but varying N or, equivalently, Z). Hence the binding energy gets maximal for N = Z. If we expand (5) in the difference N − Z we obtain ...
... This average kinetic energy has a minimum at N = Z for fixed mass number A (but varying N or, equivalently, Z). Hence the binding energy gets maximal for N = Z. If we expand (5) in the difference N − Z we obtain ...
Kinetics - A Study o..
... • the molecules must collide; • they must be positioned so that the reacting groups are together in a transition state between reactants and products; • and the collision must have enough energy to form the transition state and convert it into products. ...
... • the molecules must collide; • they must be positioned so that the reacting groups are together in a transition state between reactants and products; • and the collision must have enough energy to form the transition state and convert it into products. ...
Chapter 2 Powerpoint
... (Phase changes, volume changes) Chemical change, chemical reaction- change in matter in which new substances are formed in the product. (Combustion) Nuclear change-nuclei of one isotope spontaneously changes or is made to change into nuclei of a different isotope. ...
... (Phase changes, volume changes) Chemical change, chemical reaction- change in matter in which new substances are formed in the product. (Combustion) Nuclear change-nuclei of one isotope spontaneously changes or is made to change into nuclei of a different isotope. ...
PHY492: Nuclear & Particle Physics Lecture 5 Angular momentum Nucleon magnetic moments
... Five terms (+ means weaker binding) in a prediction of the B.E. – r ~A1/3, Binding is short ranged, depending only on nearest neighbors. This leads to a B.E. term proportional to A: –a1 A. – The surface nucleons are not surrounded by others. This leads to a term proportional to A2/3 that weakens the ...
... Five terms (+ means weaker binding) in a prediction of the B.E. – r ~A1/3, Binding is short ranged, depending only on nearest neighbors. This leads to a B.E. term proportional to A: –a1 A. – The surface nucleons are not surrounded by others. This leads to a term proportional to A2/3 that weakens the ...
Chapter 11 The Nucleus
... Split uranium-235 into two lighter nuclei; the difference in binding energy between uranium238 and the lighter nuclei is about 0.8 MeV per nucleon. Multiply this by the total number of nucleons involved, 235, and you get 188 MeV released in the fission. This is a huge amount of energy. Here's a fusi ...
... Split uranium-235 into two lighter nuclei; the difference in binding energy between uranium238 and the lighter nuclei is about 0.8 MeV per nucleon. Multiply this by the total number of nucleons involved, 235, and you get 188 MeV released in the fission. This is a huge amount of energy. Here's a fusi ...
Nuclear physics α −
... Discovery of the facts that 200 MeV of energy is released when uranium undergoes fission and other neutrons are liberated during fission, suggested the possibility of chain reaction that is a self-sustaining series of events. The reaction can be either rapid (as in a nuclear bomb) or controlled (as ...
... Discovery of the facts that 200 MeV of energy is released when uranium undergoes fission and other neutrons are liberated during fission, suggested the possibility of chain reaction that is a self-sustaining series of events. The reaction can be either rapid (as in a nuclear bomb) or controlled (as ...
PracticeSolutions - Phenix at Vanderbilt
... The external magnetic field makes the energy levels in the nucleus split according to their spin. Thus when nuclei flip their spin from one direction to another, they will jump between energy levels and emit (or absorb) photons. If we want to obtain position information on where exactly the emission ...
... The external magnetic field makes the energy levels in the nucleus split according to their spin. Thus when nuclei flip their spin from one direction to another, they will jump between energy levels and emit (or absorb) photons. If we want to obtain position information on where exactly the emission ...
Radioactivityunit6
... but we end up putting in more energy than we get out. • The left over products of fusion are relatively safe, which is why a lot of research is going into developing fusion reactors. ...
... but we end up putting in more energy than we get out. • The left over products of fusion are relatively safe, which is why a lot of research is going into developing fusion reactors. ...
Nuclear Physics and Radioactivity
... beta particle - high speed electron emitted from a radioactive element when a neutron. decays into a proton binding energy – the energy required to completely separate the nucleus into its individual protons and neutrons. element - a substance made of only one kind of atom. isotope - a form of an el ...
... beta particle - high speed electron emitted from a radioactive element when a neutron. decays into a proton binding energy – the energy required to completely separate the nucleus into its individual protons and neutrons. element - a substance made of only one kind of atom. isotope - a form of an el ...
View - Rutgers Physics
... with C4 = 23.6 MeV. It is called semiempirical because each term has a theoretical explanation but the constants are not calculated theoretically but are instead determined by a fit to the actual nuclear masses. Two other things the independent particle model gives us, if we consider it a bit more c ...
... with C4 = 23.6 MeV. It is called semiempirical because each term has a theoretical explanation but the constants are not calculated theoretically but are instead determined by a fit to the actual nuclear masses. Two other things the independent particle model gives us, if we consider it a bit more c ...
t 1/2
... Figure 13.13: Van de Graaff accelerator. Nuclei are accelerated by a high-voltage (9 million volts) terminal located within each of the cylindrical tanks. The accelerated particles travel within an evacuated beam tube (shown emerging from the tank). In the foreground is an electromagnet that deflec ...
... Figure 13.13: Van de Graaff accelerator. Nuclei are accelerated by a high-voltage (9 million volts) terminal located within each of the cylindrical tanks. The accelerated particles travel within an evacuated beam tube (shown emerging from the tank). In the foreground is an electromagnet that deflec ...
Period 1 - ND
... Use the following information to answer the next ten questions. A scanning electron microscope (SEM) is a microscope that uses a beam of electrons rather than visible light to produce images of specimens. Description of the Operation of an SEM Electrons are accelerated from the electron gun to the a ...
... Use the following information to answer the next ten questions. A scanning electron microscope (SEM) is a microscope that uses a beam of electrons rather than visible light to produce images of specimens. Description of the Operation of an SEM Electrons are accelerated from the electron gun to the a ...
12_physics_notes_ch13_nuclei
... • Binding Energy: a) It may be defined as the energy required to break a nucleus into its constituent protons and neutrons and to separate them to such a large distance that they may not interact with each ...
... • Binding Energy: a) It may be defined as the energy required to break a nucleus into its constituent protons and neutrons and to separate them to such a large distance that they may not interact with each ...
File
... Because of the existence of so many isotopes, the term element is sometimes confusing. The term nuclide is better. A nuclide is an atom that has a definite mass number A and Z-number. A list of ...
... Because of the existence of so many isotopes, the term element is sometimes confusing. The term nuclide is better. A nuclide is an atom that has a definite mass number A and Z-number. A list of ...
Basics of Nuclear Physics and Fission
... Each quantum, or unit, of a gamma ray (or other electromagnetic energy) is called a photon. Gamma rays are like light, except that they are much higher frequency electromagnetic rays. Photon energy is directly proportional to the frequency of the electromagnetic radiation. Photons of gamma rays can ...
... Each quantum, or unit, of a gamma ray (or other electromagnetic energy) is called a photon. Gamma rays are like light, except that they are much higher frequency electromagnetic rays. Photon energy is directly proportional to the frequency of the electromagnetic radiation. Photons of gamma rays can ...
Chemistry: Nuclear Reactions Guided Inquiry + n → + + 3 n +
... have 4 hydrogen atoms and 2 oxygen atoms. Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable ...
... have 4 hydrogen atoms and 2 oxygen atoms. Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable ...
Chapter 25 Nuclear Chemistry
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
Lecture 1 - Asimow.com
... about abundance of species in the Si-to-Fe range, and provides a natural mechanism for the high nuclear binding energy of the Fe group to be translated into the peak in the solar abundance pattern This particular model shows a prediction of abundance after 10 seconds of Si-burning at a temperature o ...
... about abundance of species in the Si-to-Fe range, and provides a natural mechanism for the high nuclear binding energy of the Fe group to be translated into the peak in the solar abundance pattern This particular model shows a prediction of abundance after 10 seconds of Si-burning at a temperature o ...
Nuclear fusion

In nuclear physics, nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new nucleus. During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy). Fusion is the process that powers active or ""main sequence"" stars.The fusion of two nuclei with lower masses than Iron-56 (which, along with Nickel-62, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements. There are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernova.Following the discovery of quantum tunneling by Friedrich Hund, in 1929 Robert Atkinson and Fritz Houtermans used the measured masses of light elements to predict that large amounts of energy could be released by fusing small nuclei. Building upon the nuclear transmutation experiments by Ernest Rutherford, carried out several years earlier, the laboratory fusion of hydrogen isotopes was first accomplished by Mark Oliphant in 1932. During the remainder of that decade the steps of the main cycle of nuclear fusion in stars were worked out by Hans Bethe. Research into fusion for military purposes began in the early 1940s as part of the Manhattan Project. Fusion was accomplished in 1951 with the Greenhouse Item nuclear test. Nuclear fusion on a large scale in an explosion was first carried out on November 1, 1952, in the Ivy Mike hydrogen bomb test.Research into developing controlled thermonuclear fusion for civil purposes also began in earnest in the 1950s, and it continues to this day. The present article is about the theory of fusion. For details of the quest for controlled fusion and its history, see the article Fusion power.