Short Intense Laser Pulse Collapse in Near
... gas profile as in Fig. 1 and about three times more energy in the short pulse (∼ 100 TW laser), an ion beam in the longitudinal direction should be detected reproducibly, as the laser pulse will collapse later in the jet, implying higher hot electron density current at the back and a strong longitud ...
... gas profile as in Fig. 1 and about three times more energy in the short pulse (∼ 100 TW laser), an ion beam in the longitudinal direction should be detected reproducibly, as the laser pulse will collapse later in the jet, implying higher hot electron density current at the back and a strong longitud ...
RAM Mixed Waste Disposal
... 6. Separate liquids from solids. If solids cannot be separated from liquids, affix a note to the waste container stating the composition of the solid. 7. Segregate sharps into a sharps container, disposing of full container in normal solid radioactive waste stream. 8. Radioactive wastes and organic ...
... 6. Separate liquids from solids. If solids cannot be separated from liquids, affix a note to the waste container stating the composition of the solid. 7. Segregate sharps into a sharps container, disposing of full container in normal solid radioactive waste stream. 8. Radioactive wastes and organic ...
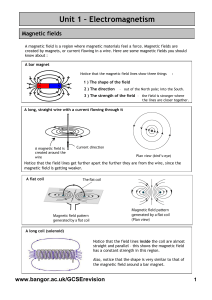
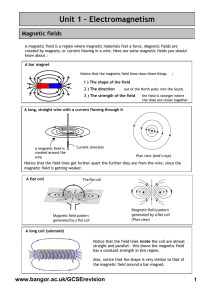
2.1 Fundamentals of Magnetism The magnetic
... material is in the unmagnetized state, the domains are nearly randomly organized and the net magnetic field for the part as a whole is zero. When a magnetizing force is applied, the domains become aligned to produce a strong magnetic field within the part. Ferromagnetic materials get their magnetic ...
... material is in the unmagnetized state, the domains are nearly randomly organized and the net magnetic field for the part as a whole is zero. When a magnetizing force is applied, the domains become aligned to produce a strong magnetic field within the part. Ferromagnetic materials get their magnetic ...
Behavior of the chemical potential/molar free energy in single
... conditions- particularly temperature. Solids melt, liquids boil, and solids can sublimate directly to vapor. ...
... conditions- particularly temperature. Solids melt, liquids boil, and solids can sublimate directly to vapor. ...
Lecture material
... Important in gaseous detectors working in one of two modes : - ionization detectors. Primary ionization from passing particle has to be drifted somehow to “sense device” where it has to be collected and measured. We would like to know what will be the spatial size of the ionization cloud, when will ...
... Important in gaseous detectors working in one of two modes : - ionization detectors. Primary ionization from passing particle has to be drifted somehow to “sense device” where it has to be collected and measured. We would like to know what will be the spatial size of the ionization cloud, when will ...
Chapter 5
... (large) porous barrier into another container • The rates of both of both processes are inversely proportional to the molar mass of the gas • Gaseous diffusion used to separate 235U from 238U in World War II CHEM 1310 A/B Fall 2006 ...
... (large) porous barrier into another container • The rates of both of both processes are inversely proportional to the molar mass of the gas • Gaseous diffusion used to separate 235U from 238U in World War II CHEM 1310 A/B Fall 2006 ...
Conversion Factors
... Label each of the following as PC (physical change), CC (chemical change), PP (physical property), or CP (chemical property). 16. ice melting 21.sugar tastes sweet 17. baking a cake 22.New substances are produced 18.silver tarnishes when exposed to air 23. No new substances are produced 19. hydrogen ...
... Label each of the following as PC (physical change), CC (chemical change), PP (physical property), or CP (chemical property). 16. ice melting 21.sugar tastes sweet 17. baking a cake 22.New substances are produced 18.silver tarnishes when exposed to air 23. No new substances are produced 19. hydrogen ...
Midterm Study Guide with Answers
... Lavoisier helped transform chemistry from a science of observation to a science of measurement. Toward this end, he developed a balance that could measure small differences in mass. He was part of a general movement toward basing conclusions on experimental evidence. PTS: 1 DIF: L2 REF: p. 15 OBJ: 1 ...
... Lavoisier helped transform chemistry from a science of observation to a science of measurement. Toward this end, he developed a balance that could measure small differences in mass. He was part of a general movement toward basing conclusions on experimental evidence. PTS: 1 DIF: L2 REF: p. 15 OBJ: 1 ...
Principle of Formation of Magnetic Field of Iron
... the magnetic field of each magnet gets on the rotating sphere of protons of another magnet. As a result of it, the protons which have a identical direction of orbital motion and rotation, are moving together with the all body under action of magnetic field, if this field is directed perpendicularly ...
... the magnetic field of each magnet gets on the rotating sphere of protons of another magnet. As a result of it, the protons which have a identical direction of orbital motion and rotation, are moving together with the all body under action of magnetic field, if this field is directed perpendicularly ...
Antiferromagnetic resonance in frustrated system Ni5(TeO3)4Br2
... turn until the applied field exceeds critical value HSF. At that point magnetic moments snap into different configuration - spin flop. Beyond this point the magnetic moment act as the filed was perpendicular to them. ...
... turn until the applied field exceeds critical value HSF. At that point magnetic moments snap into different configuration - spin flop. Beyond this point the magnetic moment act as the filed was perpendicular to them. ...
Nature of the anomalies in the supercooled liquid state of the mW
... which accounts for the finite fraction f4H of four-coordinated molecules in the high-temperature liquid, and the fraction f4L < 1 of four-coordinated molecules in the low-temperature liquid. Both f4H and f4L are estimated by an extrapolation of the fraction f4 to high and low temperature. Below Ti , ...
... which accounts for the finite fraction f4H of four-coordinated molecules in the high-temperature liquid, and the fraction f4L < 1 of four-coordinated molecules in the low-temperature liquid. Both f4H and f4L are estimated by an extrapolation of the fraction f4 to high and low temperature. Below Ti , ...
DrifterParameters.V6
... the small Electric Field region. Note that 1000 V/mm is the operating point of many liquid argon calorimeters. Molecular Oxygen ion mobility We assume that oxygen dissolved in liquid argon does not dissociate. When an oxygen molecule, a contaminant in the liquid argon, attaches an electron it become ...
... the small Electric Field region. Note that 1000 V/mm is the operating point of many liquid argon calorimeters. Molecular Oxygen ion mobility We assume that oxygen dissolved in liquid argon does not dissociate. When an oxygen molecule, a contaminant in the liquid argon, attaches an electron it become ...
Slide 1
... GEM (Gaseous Electron Multiplier) detectors utilize an electron avalanche to detect charged particles with high spatial precision. The initial particle (in our case a cosmic ray muon) enters the system and ionizes the gas, setting a minimal number of electrons free. In the presence of an electric fi ...
... GEM (Gaseous Electron Multiplier) detectors utilize an electron avalanche to detect charged particles with high spatial precision. The initial particle (in our case a cosmic ray muon) enters the system and ionizes the gas, setting a minimal number of electrons free. In the presence of an electric fi ...
The use of quantities, units and symbols in fluid inclusion research
... expressing the values of dimensionless quantities in this way, the symbol % should be used rather than the name “percent”. When any of the terms % and ppm are used it is important to state the ...
... expressing the values of dimensionless quantities in this way, the symbol % should be used rather than the name “percent”. When any of the terms % and ppm are used it is important to state the ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).