lecture ch1-3 chem161pikul
... – For charge neutrality, number of electrons and protons must be equal Atomic Symbols = Summarize information about subatomic particles – Every isotope defined by two numbers Z and A Ex. What is the atomic symbol for helium? ...
... – For charge neutrality, number of electrons and protons must be equal Atomic Symbols = Summarize information about subatomic particles – Every isotope defined by two numbers Z and A Ex. What is the atomic symbol for helium? ...
CHAPTER TWO ATOMS, MOLECULES, AND IONS For Review 1. a
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different ion ...
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different ion ...
Investigations at High Temperature in Both Equilibrium and Kinetic
... N2+, N+, O2+, O+, Al2+ and Al2O+ were registrated in the mass spectrum as representatives of the gas species over AlN. These species are established in equilibrium state, but they need not to be the only species in a kinetic orientated state. The process conditions of the formation of AlN (T>2000 °C ...
... N2+, N+, O2+, O+, Al2+ and Al2O+ were registrated in the mass spectrum as representatives of the gas species over AlN. These species are established in equilibrium state, but they need not to be the only species in a kinetic orientated state. The process conditions of the formation of AlN (T>2000 °C ...
What is a solution?
... • Particles of liquids and gases move much more freely than do particles of solids. • When gases dissolve in gases or when liquids dissolve in liquids, this movement spreads solutes evenly throughout the solvent, resulting in a homogenous solution. ...
... • Particles of liquids and gases move much more freely than do particles of solids. • When gases dissolve in gases or when liquids dissolve in liquids, this movement spreads solutes evenly throughout the solvent, resulting in a homogenous solution. ...
POLYMORPHISM (AS A PART OF PREFORMULATION STUDY)
... 4. Recent studies done on paracetamol.It consist of form-1 (monoclinic) and form-2 (orthorhombic) At high temp(500k)form-II is stable 5. Leflunomide Form I and Form II.Here Form I : Stable below transition temperature. (127c) (B)Photostability:Generally light sensitive drugs are protected from th ...
... 4. Recent studies done on paracetamol.It consist of form-1 (monoclinic) and form-2 (orthorhombic) At high temp(500k)form-II is stable 5. Leflunomide Form I and Form II.Here Form I : Stable below transition temperature. (127c) (B)Photostability:Generally light sensitive drugs are protected from th ...
AP CHEMISTRY COURSE SYLLABUS
... This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions of subject matter learned in general chemistry as well so ...
... This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions of subject matter learned in general chemistry as well so ...
Исследование вещественного состава и органического
... E-mail: [email protected] It is known that the oil reservoir is a complex heterogeneous mineral-organic system, located in the natural conditions of physical and chemical equilibrium. The introduction of active impact techniques in the field, in particular thermal and chemical, of course is penaliz ...
... E-mail: [email protected] It is known that the oil reservoir is a complex heterogeneous mineral-organic system, located in the natural conditions of physical and chemical equilibrium. The introduction of active impact techniques in the field, in particular thermal and chemical, of course is penaliz ...
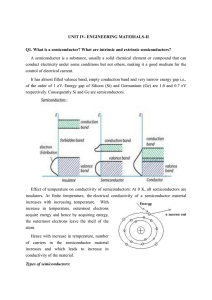
UNIT IV
... mixture is mixed with a calculated amount of phosphine (PH3) and for p-type doping; Diborane (B2H6) is employed. Diffusion technique: Involves in conversion of a region of semiconductor material by solid or gaseous diffusion of impurity atom into the crystal lattice of the semiconductor material wit ...
... mixture is mixed with a calculated amount of phosphine (PH3) and for p-type doping; Diborane (B2H6) is employed. Diffusion technique: Involves in conversion of a region of semiconductor material by solid or gaseous diffusion of impurity atom into the crystal lattice of the semiconductor material wit ...
... kinetics were conducted in synthetic gastric fluid medium (pH=1.2), and the process was found to be controlled by transient diffusion, with constant diffusivities, for the liquid into and medical agent (MA) out of the galenic forms. According to Fick’s law, a mathematical treatment led to evaluate t ...
PDF only - at www.arxiv.org.
... systems is that by increasing Y-concentration only the effect of geometrical mechanisms and thus J2 are expected to influence the phase diagram, enabling to determine their role in defining the nature and number of different phases, including the existence of ferroelectricity in modulated magnetic s ...
... systems is that by increasing Y-concentration only the effect of geometrical mechanisms and thus J2 are expected to influence the phase diagram, enabling to determine their role in defining the nature and number of different phases, including the existence of ferroelectricity in modulated magnetic s ...
Superconductivity Syllabus Col. 3
... (4) Cathode rays cause phosphorescent materials to give off light. This also shows that the cathode ray carries energy and can do work. (5) Although there was some speculation that the cathode rays were negatively charged, it is not shown to be true by experiment until 1895, just two years before Th ...
... (4) Cathode rays cause phosphorescent materials to give off light. This also shows that the cathode ray carries energy and can do work. (5) Although there was some speculation that the cathode rays were negatively charged, it is not shown to be true by experiment until 1895, just two years before Th ...
Topic 1 - BluWiki
... the one at which he is oscillating o That is why we can get him swinging very high in a relatively short time if we do this (as opposed to, say, pushing him back before he has reached the height of his swing) How does resonance apply to breaking a wine glass with sound? o It applies because if we pl ...
... the one at which he is oscillating o That is why we can get him swinging very high in a relatively short time if we do this (as opposed to, say, pushing him back before he has reached the height of his swing) How does resonance apply to breaking a wine glass with sound? o It applies because if we pl ...
Discharge Generation of Atomic Iodine
... donor used), atoms of buffer gas, and also different molecules: I2 , HI, H2 , CH3 , F2 , and some more. Atomic spectra are much simpler than molecular spectra due to a fact that molecule can rotate around its axes and distances between atoms in molecule can change (vibrations). On the other hand, th ...
... donor used), atoms of buffer gas, and also different molecules: I2 , HI, H2 , CH3 , F2 , and some more. Atomic spectra are much simpler than molecular spectra due to a fact that molecule can rotate around its axes and distances between atoms in molecule can change (vibrations). On the other hand, th ...
From Path Integrals to Fractional Quantum Statistics
... positions the many bodies can have. This is usually denoted ψ(~x1 , ~x2 , . . . , ~xN ), implying that the wavefunction is a function of ordered N -tuples of positions. However, we know that there is a hidden constraint. If the particles are indistinguishable, we must have ψ(~x1 , ~x2 , . . . , ~xN ...
... positions the many bodies can have. This is usually denoted ψ(~x1 , ~x2 , . . . , ~xN ), implying that the wavefunction is a function of ordered N -tuples of positions. However, we know that there is a hidden constraint. If the particles are indistinguishable, we must have ψ(~x1 , ~x2 , . . . , ~xN ...
Positron collisions with Rydberg atoms in strong
... give results for the angular distribution of Ps that result from a charge transfer. Finally, we investigated how large an E-field the Ps could survive depending on their emergence angle. 3.1. Single e+ collisions In this section, we investigate the cross sections and rates for the case when the atom ...
... give results for the angular distribution of Ps that result from a charge transfer. Finally, we investigated how large an E-field the Ps could survive depending on their emergence angle. 3.1. Single e+ collisions In this section, we investigate the cross sections and rates for the case when the atom ...
Synthesis and crystal structure of
... 15 M. Lattman and A.H. Cowley, Inorg. Chem., 23 (1984) 241. 16 E. Canadell, 0. Eisenstein, and J. Rubio, Organometallics, 3 (1984) 759. 17 M.B. Freeman, L.C. Sneddon, and J.C. Huffman, J. Am. Chem. Sot., 99 (1977) 5194. 18 J.F. Berar, G. CaIvarin, C. Pommier, and D. Weigel, J. Appl. Cryst., 8 (1975) ...
... 15 M. Lattman and A.H. Cowley, Inorg. Chem., 23 (1984) 241. 16 E. Canadell, 0. Eisenstein, and J. Rubio, Organometallics, 3 (1984) 759. 17 M.B. Freeman, L.C. Sneddon, and J.C. Huffman, J. Am. Chem. Sot., 99 (1977) 5194. 18 J.F. Berar, G. CaIvarin, C. Pommier, and D. Weigel, J. Appl. Cryst., 8 (1975) ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).