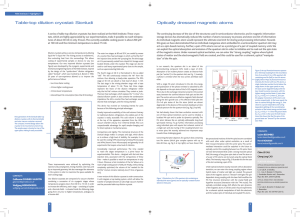
Bourdel-3 (doc, 273 KiB)
... expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes place. A low temperature can be obtained by using a combination of laser cooling and evaporative cooling te ...
... expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes place. A low temperature can be obtained by using a combination of laser cooling and evaporative cooling te ...
A deterministic source of entangled photons
... • The photon can be absorbed by the cavity mirrors, or it can be scattered into “undesired” modes of the continuum • These loss mechanisms represent a supplementary decay channel for the cavity mode, with decay rate ka • It is evident that the probability to produce the desired wave packet in each ...
... • The photon can be absorbed by the cavity mirrors, or it can be scattered into “undesired” modes of the continuum • These loss mechanisms represent a supplementary decay channel for the cavity mode, with decay rate ka • It is evident that the probability to produce the desired wave packet in each ...
Atomic Emission Spectra Quiz
... 3. Which statement correctly describes the energy that is absorbed by an atom? a) Any amount of energy can be absorbed by an atom. b) Only energies below the threshold energy can be absorbed by an atom. c) The energy that can be absorbed by an atom is quantized. d) Atoms of an element can only absor ...
... 3. Which statement correctly describes the energy that is absorbed by an atom? a) Any amount of energy can be absorbed by an atom. b) Only energies below the threshold energy can be absorbed by an atom. c) The energy that can be absorbed by an atom is quantized. d) Atoms of an element can only absor ...
Bose-Einstein Condensation
... • The fastest moving atoms move furthest from the minimum, to a position of highest energy (see the upper atom shown in the figure). • Magnetic resonance is used to reverse the moments of the most energetic atoms, causing them to leave the trap, which is now an energy maximum. • Slowly reducing the ...
... • The fastest moving atoms move furthest from the minimum, to a position of highest energy (see the upper atom shown in the figure). • Magnetic resonance is used to reverse the moments of the most energetic atoms, causing them to leave the trap, which is now an energy maximum. • Slowly reducing the ...
Atomic Spectra - Northeast High School
... For other atoms, the energies cannot be so easily calculated, and will often be given to you directly. ...
... For other atoms, the energies cannot be so easily calculated, and will often be given to you directly. ...
L34
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
L 35 Modern Physics [1]
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
Problem set VI Problem 6.1 Problem 6.2 Problem 6.3 Problem 6.4
... direction, i.e. |Sx , +i. When this beam goes through a Stern-Gerlach apparatus with an inhomogeneous magnetic field in the z-direction (SGz), it splits into two beams of equal intensity, i.e. | h+| Sx , +i |2 = | h−| Sx , +i |2 = ...
... direction, i.e. |Sx , +i. When this beam goes through a Stern-Gerlach apparatus with an inhomogeneous magnetic field in the z-direction (SGz), it splits into two beams of equal intensity, i.e. | h+| Sx , +i |2 = | h−| Sx , +i |2 = ...
Exercice N°1 : Laser à 4 niveaux
... We consider a Nd:YVO4 laser pumped in continuous wave either at 808 nm or at 914 nm (fig.1). The laser effect occurs at 1064 nm. With a pumping at 808 nm, the Nd:YVO4 is a four level system. The transitions 0-3, 0-2, and 1-2 are purely radiative whereas the transitions 3-2 and 1-0 are non radiative ...
... We consider a Nd:YVO4 laser pumped in continuous wave either at 808 nm or at 914 nm (fig.1). The laser effect occurs at 1064 nm. With a pumping at 808 nm, the Nd:YVO4 is a four level system. The transitions 0-3, 0-2, and 1-2 are purely radiative whereas the transitions 3-2 and 1-0 are non radiative ...
Topics 2 and 12 Outline
... Developments in scientific research follow improvements in apparatus—the use of electricity and magnetism in Thomson’s cathode rays. (1.8) Theories being superseded—quantum mechanics is among the most current models of the atom. (1.9) Use theories to explain natural phenomena—line spectra explained ...
... Developments in scientific research follow improvements in apparatus—the use of electricity and magnetism in Thomson’s cathode rays. (1.8) Theories being superseded—quantum mechanics is among the most current models of the atom. (1.9) Use theories to explain natural phenomena—line spectra explained ...
extra information - Patrick Tevlin Music
... The lamp must be turned on. Just turn it on slightly. The energy of the photons must exactly match the difference in energy between the two levels. This is very different from what happens when you excite atoms with electrons. Electrons must have at least the exact energy. They can have more tha ...
... The lamp must be turned on. Just turn it on slightly. The energy of the photons must exactly match the difference in energy between the two levels. This is very different from what happens when you excite atoms with electrons. Electrons must have at least the exact energy. They can have more tha ...













![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001689016_1-3e506855e2f70cb00e132a79d00855e2-300x300.png)