Center for Biological Physics* Math and Science Teachers Fellows
... interaction of amino acid side chains. Recognize areas of rigidity and flexibility in proteins. Flexible areas interact with other molecules to undergo chemical reactions. Great potential for combating disease as proteins could be manufactured for use. ♦ Use of computer simulations in analyzing data ...
... interaction of amino acid side chains. Recognize areas of rigidity and flexibility in proteins. Flexible areas interact with other molecules to undergo chemical reactions. Great potential for combating disease as proteins could be manufactured for use. ♦ Use of computer simulations in analyzing data ...
Macromolecules Part 2
... 3. Alpha (α) Carbon – This is the central Carbon that holds the whole molecule together. 4. R group - This is the most important part as it gives each amino acid its distinctly different property. (Notice all 20 amino acids have a different R group.) E. Individual Amino Acids (monomers) are bonded t ...
... 3. Alpha (α) Carbon – This is the central Carbon that holds the whole molecule together. 4. R group - This is the most important part as it gives each amino acid its distinctly different property. (Notice all 20 amino acids have a different R group.) E. Individual Amino Acids (monomers) are bonded t ...
Protein and Enzyme Check for Understanding
... Protein and Enzyme Check for Understanding: 1. What is the monomer of a protein? 2. What is the name of the bond between the amino acids in a protein? 3. Label the following parts: ...
... Protein and Enzyme Check for Understanding: 1. What is the monomer of a protein? 2. What is the name of the bond between the amino acids in a protein? 3. Label the following parts: ...
Protein Purification and Analysis Day 4
... will also vary with the nature of this conformation (higher mobility for more compact conformations, lower for larger structures like oligomers). If native PAGE is carried out near neutral pH to avoid acid or alkaline denaturation, then it can be used to study conformation, self-association or aggre ...
... will also vary with the nature of this conformation (higher mobility for more compact conformations, lower for larger structures like oligomers). If native PAGE is carried out near neutral pH to avoid acid or alkaline denaturation, then it can be used to study conformation, self-association or aggre ...
Chapter 4 - Open Yale Courses
... shape is the tertiary structure, and the formation of a complex with other polypeptide chains is the quaternary structure. • All the information necessary for a protein to fold properly into its tertiary structure is contained in the primary amino acid sequence. • Non-covalent interactions such as h ...
... shape is the tertiary structure, and the formation of a complex with other polypeptide chains is the quaternary structure. • All the information necessary for a protein to fold properly into its tertiary structure is contained in the primary amino acid sequence. • Non-covalent interactions such as h ...
RNA and protein synthesis
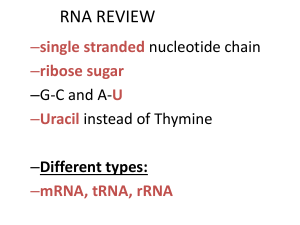
... Protein synthesis occurs in two major parts transcription and translation. 1. Transcription: Process where DNA serves as a template to produce complementary mRNA 2. Translation: Process in which mRNA is used to link amino acids together to synthesize proteins. Involves tRNA and rRNA DNA ...
... Protein synthesis occurs in two major parts transcription and translation. 1. Transcription: Process where DNA serves as a template to produce complementary mRNA 2. Translation: Process in which mRNA is used to link amino acids together to synthesize proteins. Involves tRNA and rRNA DNA ...
Oct - CSIR-NEIST, Jorhat
... 3DNALandscapes, located at: http://3DNAscapes.rutgers.edu, is a new database for exploring the conformational features of DNA. In contrast to most structural databases, which archive the Cartesian coordinates and/or derived parameters and images for individual structures, 3DNALandscapes enables sear ...
... 3DNALandscapes, located at: http://3DNAscapes.rutgers.edu, is a new database for exploring the conformational features of DNA. In contrast to most structural databases, which archive the Cartesian coordinates and/or derived parameters and images for individual structures, 3DNALandscapes enables sear ...
Biological Macromolecules
... **Antibodies are part of the immune system. **When something enters the body that isn’t supposed to be there, like certain bacteria, antibodies find the invader and stick themselves onto it. **White Blood cells destroy the invaders (hopefully) ...
... **Antibodies are part of the immune system. **When something enters the body that isn’t supposed to be there, like certain bacteria, antibodies find the invader and stick themselves onto it. **White Blood cells destroy the invaders (hopefully) ...
An Approach to Including Protein Quality When
... The production of protein from animal sources is often criticized because of the low efficiency of converting plant protein from feeds into protein in the animal products. However, this critique does not consider the fact that large portions of the plant-based proteins fed to animals may be human-in ...
... The production of protein from animal sources is often criticized because of the low efficiency of converting plant protein from feeds into protein in the animal products. However, this critique does not consider the fact that large portions of the plant-based proteins fed to animals may be human-in ...
Structures define the functions of proteins
... This can be explained by chaperones. (above 85% protein folding) - Molecular chaperone : bind to exposed hydrophobic regions and stabilize them, thereby preventing these proteins from aggregating and being degraded. (Hsp70+Hsp40, GrpE+DnaK) - Chaperonins directly facilitate the folding of proteins ( ...
... This can be explained by chaperones. (above 85% protein folding) - Molecular chaperone : bind to exposed hydrophobic regions and stabilize them, thereby preventing these proteins from aggregating and being degraded. (Hsp70+Hsp40, GrpE+DnaK) - Chaperonins directly facilitate the folding of proteins ( ...
Biochemistry Course #: - College of Pharmacy at Howard University
... Factors affecting the Stability of the α - helices 1. The electrostatic repulsion or attraction between successive amino acid residues with charged R groups. 2. The bulkiness of the adjacent R groups 3. The interactions between R groups spaced 3 or 4 residues apart. 4. The occurrence of Pro and Gly ...
... Factors affecting the Stability of the α - helices 1. The electrostatic repulsion or attraction between successive amino acid residues with charged R groups. 2. The bulkiness of the adjacent R groups 3. The interactions between R groups spaced 3 or 4 residues apart. 4. The occurrence of Pro and Gly ...
Unit 3 Biochemistry - The Naked Science Society
... The most common elements found in living organisms include: ...
... The most common elements found in living organisms include: ...
Appendices Enzyme Endurance Review of Protein Structure Great
... precisely positioned so that they favor the formation of the high-energy transition states that the substrates must pass through. ...
... precisely positioned so that they favor the formation of the high-energy transition states that the substrates must pass through. ...
Proteins & Nucleic Acids - St. Mary Catholic Secondary School
... Ladder shape – Rails - A series of alternating phosphates and sugars linked by covalent bonds known as phosphodiester bonds. Rungs of the ladder are made of the nitrogenous bases and their hydrogen bonds. The nitrogenous bases involved with DNA are adenine, cytosine, guanine and thymine. The adenine ...
... Ladder shape – Rails - A series of alternating phosphates and sugars linked by covalent bonds known as phosphodiester bonds. Rungs of the ladder are made of the nitrogenous bases and their hydrogen bonds. The nitrogenous bases involved with DNA are adenine, cytosine, guanine and thymine. The adenine ...
Enzyme Biosinthess
... Posttranslational Processing of Proteins Folding Amino acid modification (some proteins) Proteolytic cleavage ...
... Posttranslational Processing of Proteins Folding Amino acid modification (some proteins) Proteolytic cleavage ...
Lecture 1: Fundamentals of Protein Structure
... Primary sequence reveals important clues about a protein • Evolution conserves amino acids that are important to protein structure and function across species. Sequence comparison of multiple “homologs” of a particular protein reveals highly conserved regions that are important for function. • Clus ...
... Primary sequence reveals important clues about a protein • Evolution conserves amino acids that are important to protein structure and function across species. Sequence comparison of multiple “homologs” of a particular protein reveals highly conserved regions that are important for function. • Clus ...
ANSWERS TO REVIEW QUESTIONS – CHAPTER 02
... Fibrous and globular proteins can be distinguished by their structures. The primary and secondary structures of proteins refer respectively to (1) the sequence of amino acid monomers and (2) the bending and folding of the amino acid chain. The tertiary structure of a protein refers to the overall sh ...
... Fibrous and globular proteins can be distinguished by their structures. The primary and secondary structures of proteins refer respectively to (1) the sequence of amino acid monomers and (2) the bending and folding of the amino acid chain. The tertiary structure of a protein refers to the overall sh ...
Protein Aggregation in High-Protein Caramel
... sugar (sucrose, corn syrup, lactose) continuous phase with fat globules homogeneously dispersed throughout. Lecithin is often used to help create small fat globules, although proteins and the high-viscosity amorphous phase help prevent lipid coalescence. By varying water content, caramels can be mad ...
... sugar (sucrose, corn syrup, lactose) continuous phase with fat globules homogeneously dispersed throughout. Lecithin is often used to help create small fat globules, although proteins and the high-viscosity amorphous phase help prevent lipid coalescence. By varying water content, caramels can be mad ...
Isolation of proteins
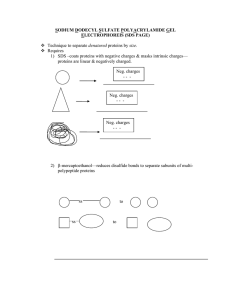
... -based on the binding of Coomassie Brilliant Blue G-250 dye to the proteins and particularly basic and aromatic amino acids residues (hydrophilic arginine (ARG) and the hydrophobic phenylalanine (PHE), tryptophan (TRY), and proline (PRO) (aromatic amino acid residues). As the Coomassie preferentiall ...
... -based on the binding of Coomassie Brilliant Blue G-250 dye to the proteins and particularly basic and aromatic amino acids residues (hydrophilic arginine (ARG) and the hydrophobic phenylalanine (PHE), tryptophan (TRY), and proline (PRO) (aromatic amino acid residues). As the Coomassie preferentiall ...
Protein misfolding associated to mild modifications of local cellular pH
... Protein misfolding associated to mild modifications of local cellular pH: Amyloid-like aggregation of human apolipoprotein A-I variants Ramella N., Tricerri M. A., Rimoldi O.J. INIBIOLP-CONICET. Fac. Ciencias Medicas, UNLP. Argentina The native folding of proteins is critical to fulfill their biolog ...
... Protein misfolding associated to mild modifications of local cellular pH: Amyloid-like aggregation of human apolipoprotein A-I variants Ramella N., Tricerri M. A., Rimoldi O.J. INIBIOLP-CONICET. Fac. Ciencias Medicas, UNLP. Argentina The native folding of proteins is critical to fulfill their biolog ...
Protein folding
... Proteins are synthesized on ribosomes as linear polypeptites. As they are synthesized they assume secondary and tertiary structure. Activity of proteins depend on the integrity of its final tertiary structure also reffered as the native form. The native form of protein is not very stable structure a ...
... Proteins are synthesized on ribosomes as linear polypeptites. As they are synthesized they assume secondary and tertiary structure. Activity of proteins depend on the integrity of its final tertiary structure also reffered as the native form. The native form of protein is not very stable structure a ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.