Western blot analysis
... query were defined using an E value cut-off of 10-4. Remote structural homology between Etk, Wzc, MinD, ParA and SopA were established by hidden Markov model (HMM)-HMM alignments using HHpred [10]. The HMM profile was generated by aligning full length protein sequences of MinD, ParA, SopA followed b ...
... query were defined using an E value cut-off of 10-4. Remote structural homology between Etk, Wzc, MinD, ParA and SopA were established by hidden Markov model (HMM)-HMM alignments using HHpred [10]. The HMM profile was generated by aligning full length protein sequences of MinD, ParA, SopA followed b ...
BIOCHEMISTRY REVIEW SHEET
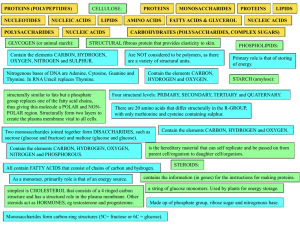
... b. What is the ratio of H to O______________________________ c. Make up an example showing this ratio.___________________________ d. What is the chemical formula of glucose?________________________________ e. Define the following terms: Monosaccharide Disaccharide Polysaccharide f. Name 1 monosaccha ...
... b. What is the ratio of H to O______________________________ c. Make up an example showing this ratio.___________________________ d. What is the chemical formula of glucose?________________________________ e. Define the following terms: Monosaccharide Disaccharide Polysaccharide f. Name 1 monosaccha ...
23. ______ layers of ______ make up the cell
... 9. Name 3 monosaccharides. 10. Monosaccharides are ___________ sugars. 11. Long chains of sugars are ______________. Name three. Proteins are made of subunits called amino acids and are used to build cells and do much of the work inside organisms. They also act as enzymes helping to control metaboli ...
... 9. Name 3 monosaccharides. 10. Monosaccharides are ___________ sugars. 11. Long chains of sugars are ______________. Name three. Proteins are made of subunits called amino acids and are used to build cells and do much of the work inside organisms. They also act as enzymes helping to control metaboli ...
Proteins
... causing it to uncoil or form a new shape. This is caused by heat, pH, or radiation. This change is not permanent Coagulation is a permanent change in the structure. Example is a boiled egg ...
... causing it to uncoil or form a new shape. This is caused by heat, pH, or radiation. This change is not permanent Coagulation is a permanent change in the structure. Example is a boiled egg ...
Introduction to Molecular forces and Macromolecules
... onto jello surface. .examine during next two hours (effect of gelatinase from plant origin on the jell-O . ...
... onto jello surface. .examine during next two hours (effect of gelatinase from plant origin on the jell-O . ...
Prof. Dr. Harry F. Noller Prof. Dr. Ada Yonath
... daughter cell has an identical set of chromosomes. Furthermore, the DNA carries all the genetic information in the cell and is responsible for coding for all the proteins, which consist of long chains of amino acids and serve as the building blocks of our body that carry out all its vital functions. ...
... daughter cell has an identical set of chromosomes. Furthermore, the DNA carries all the genetic information in the cell and is responsible for coding for all the proteins, which consist of long chains of amino acids and serve as the building blocks of our body that carry out all its vital functions. ...
Protein 101
... •How does this fit with Rx for athletes •Upper end of range clearly exceeds Rx for “athlete” Rx •*1.2-1.4 g/d /kg for endurance athletes *1.4-1.8 g/d/kg for strength athletes are adequate to support the ...
... •How does this fit with Rx for athletes •Upper end of range clearly exceeds Rx for “athlete” Rx •*1.2-1.4 g/d /kg for endurance athletes *1.4-1.8 g/d/kg for strength athletes are adequate to support the ...
Naomi`s Nucleants - Molecular Dimensions
... Naomi’s Nucleants have facilitated the crystallization of 14 proteins, the highest number reported for any single nucleant. Many of these proteins have proven difficult to crystallize and some of these, including membrane proteins, have only been crystallized in the presence of Naomi’s Nucleants. Na ...
... Naomi’s Nucleants have facilitated the crystallization of 14 proteins, the highest number reported for any single nucleant. Many of these proteins have proven difficult to crystallize and some of these, including membrane proteins, have only been crystallized in the presence of Naomi’s Nucleants. Na ...
Ch. 5 Notes
... - organic molecules possessing both carboxyl and amino groups - differ in their properties due to differing side chains, called R groups - 20 different amino acids make up proteins. 2. Amino Acid Polymers - Amino acids are linked by peptide bonds. 3. Determining the Amino Acid Sequence of a Polypept ...
... - organic molecules possessing both carboxyl and amino groups - differ in their properties due to differing side chains, called R groups - 20 different amino acids make up proteins. 2. Amino Acid Polymers - Amino acids are linked by peptide bonds. 3. Determining the Amino Acid Sequence of a Polypept ...
new proteins
... molecule and makes parts of it vibrate faster. • This means that the bonds (not co-valent) that hold the protein in its globular shape are broken and its complex shape will unravel. ...
... molecule and makes parts of it vibrate faster. • This means that the bonds (not co-valent) that hold the protein in its globular shape are broken and its complex shape will unravel. ...
BNFO 602 Lecture 1 - New Jersey Institute of Technology
... of four letters: A, C, G, and T. They can be very long, e.g. thousands and even millions of letters • Proteins are also represented as strings of 20 letters (each letter is an amino acid). Their 3-D structure determines the function to a large extent. ...
... of four letters: A, C, G, and T. They can be very long, e.g. thousands and even millions of letters • Proteins are also represented as strings of 20 letters (each letter is an amino acid). Their 3-D structure determines the function to a large extent. ...
CELL MEMBRANES CHAPTER 6 FLUID MOSAIC MODEL
... Proteins are anchored or freely movable Proteins and lipids in the membrane are independent and only interact noncovalently. Or covalently attached and are referred to as anchored membrane proteins. Some move freely This shows the fluidity of cell membranes EXTERNAL CARBOHYDRATES Cell adhesion and c ...
... Proteins are anchored or freely movable Proteins and lipids in the membrane are independent and only interact noncovalently. Or covalently attached and are referred to as anchored membrane proteins. Some move freely This shows the fluidity of cell membranes EXTERNAL CARBOHYDRATES Cell adhesion and c ...
Macromolecule Review - Mr. Dudley`s Website
... A dog gets many nutrients from its food including amino acids. Which of these can be built directly using the amino acids? ...
... A dog gets many nutrients from its food including amino acids. Which of these can be built directly using the amino acids? ...
STUDY PROBLEMS AND CALCULATIONS: UV/VIS
... 1. Describe the general principle of colorimetric assays. 2. Which chemical groups are responsible for the absorption of ultra-violet radiation in proteins? Are proteins able to absorb visible light? 3. Which chemical groups absorb UV light in nucleic acids? What is max of this absorption? 4. Altern ...
... 1. Describe the general principle of colorimetric assays. 2. Which chemical groups are responsible for the absorption of ultra-violet radiation in proteins? Are proteins able to absorb visible light? 3. Which chemical groups absorb UV light in nucleic acids? What is max of this absorption? 4. Altern ...
Slide 1 - Denton ISD
... Are NOT considered to be polymers, as there are a variety of structural units. ...
... Are NOT considered to be polymers, as there are a variety of structural units. ...
Protein Structure and Function
... - Molecular chaperones and chaperonins prevent aggregation of unfolded protein ...
... - Molecular chaperones and chaperonins prevent aggregation of unfolded protein ...
chapter 5 large biological molecules
... o Tertiary structure – 3-D irregular structure that results from bonding between side chains of the various amino acids; Types of bonding: hydrophobic interaction, Van der Waals forces, H bonds, ionic bonds, and disulfide bridges. o Quaternary structure – if it has 2 or more polypeptide chains. De ...
... o Tertiary structure – 3-D irregular structure that results from bonding between side chains of the various amino acids; Types of bonding: hydrophobic interaction, Van der Waals forces, H bonds, ionic bonds, and disulfide bridges. o Quaternary structure – if it has 2 or more polypeptide chains. De ...
Protein synthesis and Enzyme test review
... Trp, Glu, Ile Trp= UGG Glu= GAA or GAG Ile= AUU or AUC or AUA 11. mRNA has (codons / anticodons), and tRNA has (codons / anticodons). 12. What is the function of tRNA? Transfer amino acids to the ribosome ...
... Trp, Glu, Ile Trp= UGG Glu= GAA or GAG Ile= AUU or AUC or AUA 11. mRNA has (codons / anticodons), and tRNA has (codons / anticodons). 12. What is the function of tRNA? Transfer amino acids to the ribosome ...
Macromolecules Review ws Name the 6 main elements that make
... 16. Chains of amino acids make polypeptides which can join together to make a protein. 17. Phosholipids makes up cell membranes. 18. Fats are made of an alcohol called glycerol and three fatty acids chains. This is known as a triglyceride 19. If there are all SINGLE bonds between carbons in the fat ...
... 16. Chains of amino acids make polypeptides which can join together to make a protein. 17. Phosholipids makes up cell membranes. 18. Fats are made of an alcohol called glycerol and three fatty acids chains. This is known as a triglyceride 19. If there are all SINGLE bonds between carbons in the fat ...
File - Peterson Biology
... 3. tRNA brings correct amino acid (methionine) to the ribosome. Each tRNA carries one type of amino acid. The anticodon (three nitrogen bases on tRNA) must ...
... 3. tRNA brings correct amino acid (methionine) to the ribosome. Each tRNA carries one type of amino acid. The anticodon (three nitrogen bases on tRNA) must ...
Chapter Five
... Stomach acid opens up the protein’s structure and permits digestive enzymes to act upon the protein. ...
... Stomach acid opens up the protein’s structure and permits digestive enzymes to act upon the protein. ...
Lecture_11_2005
... Folds/motifs - tertiary structure • How these secondary structure elements come together to form structure. – Helix-turn-helix ...
... Folds/motifs - tertiary structure • How these secondary structure elements come together to form structure. – Helix-turn-helix ...
Study Guide for Membranes and Transport
... describe the processes which allow monomers to be joined to form polymers as well as polymers to be broken down into monomers. give examples of carbohydrates, lipids, proteins, and nucleic acids including at least one location within a cell where each can be found. compare and contrast the str ...
... describe the processes which allow monomers to be joined to form polymers as well as polymers to be broken down into monomers. give examples of carbohydrates, lipids, proteins, and nucleic acids including at least one location within a cell where each can be found. compare and contrast the str ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.