CHEMISTRY ANSWERS TO Textbook Questions
... (d) malleable, waxy, soft, cylindrical (e) shiny, conductive, silver-coloured, malleable (f) white, crystalline, sweet, soluble in water (g) soft, viscous, white, adhesive/sticky 5. (a) The dimensions of the fabric change without altering its composition. (b) The salad is a mixture of ingredients. I ...
... (d) malleable, waxy, soft, cylindrical (e) shiny, conductive, silver-coloured, malleable (f) white, crystalline, sweet, soluble in water (g) soft, viscous, white, adhesive/sticky 5. (a) The dimensions of the fabric change without altering its composition. (b) The salad is a mixture of ingredients. I ...
Chemical Reactions and The Mole Review
... • Focus question: What is the law of conservation of mass and what does it have to do with balancing chemical equations? • As you watch the video, jot down your thoughts on the focus question under your catalyst. Then, be ready to share. ...
... • Focus question: What is the law of conservation of mass and what does it have to do with balancing chemical equations? • As you watch the video, jot down your thoughts on the focus question under your catalyst. Then, be ready to share. ...
Mixture Solution Notes
... 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
... 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
atom a very small particle that makes up most kinds of matters and
... up most kinds of matters and consists of smaller parts called protons, neutrons and electrons. the basic building block of matter - the smallest particle of an element that still has all the properties of that element a number equal to the sum of the number of protons and neutron in an atom’s nucleu ...
... up most kinds of matters and consists of smaller parts called protons, neutrons and electrons. the basic building block of matter - the smallest particle of an element that still has all the properties of that element a number equal to the sum of the number of protons and neutron in an atom’s nucleu ...
Chemistry Fall-2016 Final
... AW. any metal in Group 1A of the periodic table; usually soft, shiny, and react violently with water ...
... AW. any metal in Group 1A of the periodic table; usually soft, shiny, and react violently with water ...
World Geography - Sayre Geography Class
... the most important factors. (carbonic acid – caves are formed this way) Acid rain is a type of chemical weathering caused by air pollution and water. ...
... the most important factors. (carbonic acid – caves are formed this way) Acid rain is a type of chemical weathering caused by air pollution and water. ...
7 - Amphibian Ark
... When to expect egg deposition or birth. Any special husbandry considerations during incubation or hatching, and details of artificial incubation procedures are outlined. 2.5.4 Development and Care of Eggs and Larvae * Conditions for incubation of eggs and time required for hatching is detailed here ...
... When to expect egg deposition or birth. Any special husbandry considerations during incubation or hatching, and details of artificial incubation procedures are outlined. 2.5.4 Development and Care of Eggs and Larvae * Conditions for incubation of eggs and time required for hatching is detailed here ...
Physical and Chemical Changes
... Hardness: Compare hardness of objects by seeing which one would scratch the other. ...
... Hardness: Compare hardness of objects by seeing which one would scratch the other. ...
CLASSROOM CONNECTORS
... Chemical properties are those properties a substance possesses because of its action or lack of action with other substances. Reaction with an acid, or reaction with oxygen (combustion) are just a couple of examples of chemical properties. Studying chemical properties is usually done when chemical c ...
... Chemical properties are those properties a substance possesses because of its action or lack of action with other substances. Reaction with an acid, or reaction with oxygen (combustion) are just a couple of examples of chemical properties. Studying chemical properties is usually done when chemical c ...
Balancing Chemical Equations
... • Relate the conservation of mass to the rearrangement of atoms in a chemical reaction • Write and interpret a balanced chemical equation for a reaction, and relate conservation of mass to the balanced equation ...
... • Relate the conservation of mass to the rearrangement of atoms in a chemical reaction • Write and interpret a balanced chemical equation for a reaction, and relate conservation of mass to the balanced equation ...
File - Flipped Out Science with Mrs. Thomas!
... 14. Explain why a color change when two or more substances are mixed together, does not always indicate a chemical change. Color change can also be a physical change - for example, mixing kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for examp ...
... 14. Explain why a color change when two or more substances are mixed together, does not always indicate a chemical change. Color change can also be a physical change - for example, mixing kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for examp ...
Chemistry lesson note
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
Reactions Unit Plan
... Chemical Reactions A. Identify a chemical change. (MOCLE 1.1.G.a) 1. Classify the type of chemical reaction as synthesis, decomposition, single replacement, double replacement, and combustion. 2. Predict the products of a chemical reaction using the activity series of metals and solubility rules. B. ...
... Chemical Reactions A. Identify a chemical change. (MOCLE 1.1.G.a) 1. Classify the type of chemical reaction as synthesis, decomposition, single replacement, double replacement, and combustion. 2. Predict the products of a chemical reaction using the activity series of metals and solubility rules. B. ...
Chapter 5 – Chemical Reactions
... Particle size – the smaller the particles the faster the reaction (example – dust explosion) Higher temperature – the higher the temperature the faster the reaction Increase concentration of solution (a more concentrated acid will react faster than a dilute ...
... Particle size – the smaller the particles the faster the reaction (example – dust explosion) Higher temperature – the higher the temperature the faster the reaction Increase concentration of solution (a more concentrated acid will react faster than a dilute ...
Environmental Chemistry
... The monitoring of the levels of pollutants in any ecosystem requires knowledge of how to detect those pollutants or their effects, either by chemical testing or direct observation of biological organisms. Biological indicators are organisms whose presence or absence gives clues as to the amount of p ...
... The monitoring of the levels of pollutants in any ecosystem requires knowledge of how to detect those pollutants or their effects, either by chemical testing or direct observation of biological organisms. Biological indicators are organisms whose presence or absence gives clues as to the amount of p ...
SCH3U Course Review
... decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
... decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
chemical reaction
... Determining Physical States Aqueous- dissolved in water -Many chemical reactions are occur when one or more of the reactants are dissolved in water; however not all products formed from aqueous reactions are dissolved in water -In order for a product to be aqueous it must be soluble in water; this ...
... Determining Physical States Aqueous- dissolved in water -Many chemical reactions are occur when one or more of the reactants are dissolved in water; however not all products formed from aqueous reactions are dissolved in water -In order for a product to be aqueous it must be soluble in water; this ...
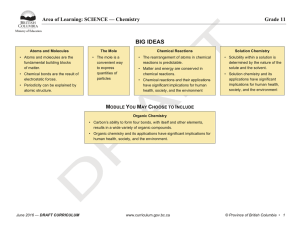
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
Matter – Properties and Changes 1 Intensive properties
... Ability of a substance to combine with or change into 1 or more substances AKA: chemical reaction – o new substance formed in the reaction that has different composition and properties from the original o Crushing grapes physical change Fermenting grape juice and sugars into wine chemical change ...
... Ability of a substance to combine with or change into 1 or more substances AKA: chemical reaction – o new substance formed in the reaction that has different composition and properties from the original o Crushing grapes physical change Fermenting grape juice and sugars into wine chemical change ...
HONORS: UNIT 2B: Antacids Below are the class objectives
... Below are the class objectives, correlated to the NC Essential Standards and page numbers within your textbook. In the far right column you will see the required vocabulary of the unit as well. At the end of each unit, you should be able to define and apply all the required vocabulary words in order ...
... Below are the class objectives, correlated to the NC Essential Standards and page numbers within your textbook. In the far right column you will see the required vocabulary of the unit as well. At the end of each unit, you should be able to define and apply all the required vocabulary words in order ...
Unit 2.2 Test Review Key
... 14. Explain why a color change when two or more substances are mixed together, does not always indicate a chemical change. Color change can also be a physical change - for example, mixing kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for examp ...
... 14. Explain why a color change when two or more substances are mixed together, does not always indicate a chemical change. Color change can also be a physical change - for example, mixing kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for examp ...
Ductility-the ability to be stretched into wires
... • Is the ability to be torn a physical or chemical property? – Physical Property: Property that can be tested/observed without changing chemical identity of the substance; can be undone ...
... • Is the ability to be torn a physical or chemical property? – Physical Property: Property that can be tested/observed without changing chemical identity of the substance; can be undone ...
The Language of Chemistry
... • When separated, the components of both types of mixtures yields pure substances. ...
... • When separated, the components of both types of mixtures yields pure substances. ...
Introductory Chemistry Test Review
... a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 + Na3PO4 11. Write a complete and balanced equation for the dissociation of the following compounds when dissolv ...
... a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 + Na3PO4 11. Write a complete and balanced equation for the dissociation of the following compounds when dissolv ...
Safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is an important component of product stewardship and occupational safety and health. It is intended to provide workers and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information such as physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. SDS formats can vary from source to source within a country depending on national requirements.SDSs are a widely used system for cataloging information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product. These data sheets can be found anywhere where chemicals are being used.There is also a duty to properly label substances on the basis of physico-chemical, health and/or environmental risk. Labels can include hazard symbols such as the European Union standard black diagonal cross on an orange background, used to denote a harmful substance.A SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting.In some jurisdictions, the SDS is required to state the chemical's risks, safety, and effect on the environment.It is important to use an SDS specific to both country and supplier, as the same product (e.g. paints sold under identical brand names by the same company) can have different formulations in different countries. The formulation and hazard of a product using a generic name (e.g. sugar soap) may vary between manufacturers in the same country.