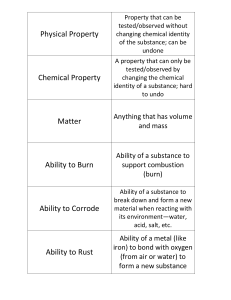
PHYSICAL PROPERTIES - can observe w/o changing the
... CHEMICAL CHANGES – alter the substance’s identity at an atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to wa ...
... CHEMICAL CHANGES – alter the substance’s identity at an atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to wa ...
Chapter 1 Chemistry: The Study of Matter
... A change that alters the appearance without changing the composition. (A change in physical properties) Examples? Boiled water is still water. Chemical changes - a change where a new form of matter is produced. Examples? Iron rusting ...
... A change that alters the appearance without changing the composition. (A change in physical properties) Examples? Boiled water is still water. Chemical changes - a change where a new form of matter is produced. Examples? Iron rusting ...
Chapter 1 Student Notes
... C. Changes of Matter Physical Property: anything you can observe without destroying or changing the composition of a substance ex) odor, color, volume, density, MP, BP Chemical Property: describes how a substance(s) can change to form a new substance Physical Change: identifying properties remain un ...
... C. Changes of Matter Physical Property: anything you can observe without destroying or changing the composition of a substance ex) odor, color, volume, density, MP, BP Chemical Property: describes how a substance(s) can change to form a new substance Physical Change: identifying properties remain un ...
(the products). Mass is conserved in a chemical reaction
... • A chemical equation uses chemical formulas to describe the chemicals that react (the reactants) and those that are produced (the products). ...
... • A chemical equation uses chemical formulas to describe the chemicals that react (the reactants) and those that are produced (the products). ...
Chemical Equations
... The products are nitrogen and hydrogen Are they diatomic or not? Yes, therefore products are N2 and H2 NH3 N2 + H2 unbalanced 2 NH3 N2 + 3 H2 balanced ...
... The products are nitrogen and hydrogen Are they diatomic or not? Yes, therefore products are N2 and H2 NH3 N2 + H2 unbalanced 2 NH3 N2 + 3 H2 balanced ...
Chemical Reactions
... - To observe some chemical reactions and identify reactants and products of those reactions. - To classify reactions as to type and write symbols showing phases. - To practice and learn the splint test for gases. ...
... - To observe some chemical reactions and identify reactants and products of those reactions. - To classify reactions as to type and write symbols showing phases. - To practice and learn the splint test for gases. ...
4:1 Part 4 Public Meetings and Freedom of Information Act § 4
... prior to the meeting. If rules were not adopted prior to the meeting, broadcasting must be permitted (Section 1-226). § 4-7. Definition of “Meeting.” A. The definition of “meeting” under the Freedom of Information Act is relatively broad. A meeting is defined, in addition to the obvious meanings, as ...
... prior to the meeting. If rules were not adopted prior to the meeting, broadcasting must be permitted (Section 1-226). § 4-7. Definition of “Meeting.” A. The definition of “meeting” under the Freedom of Information Act is relatively broad. A meeting is defined, in addition to the obvious meanings, as ...
Section 4-1
... time. Some examples of competition: crop plants and weeds compete for food, water, and sunlight; wolves and foxes compete for the same food (rabbits). ...
... time. Some examples of competition: crop plants and weeds compete for food, water, and sunlight; wolves and foxes compete for the same food (rabbits). ...
one
... – Start by balancing an element that appears in only one reactant and product. – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will chan ...
... – Start by balancing an element that appears in only one reactant and product. – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will chan ...
Physical Property
... A property that can only be tested/observed by changing the chemical identity of a substance; hard to undo ...
... A property that can only be tested/observed by changing the chemical identity of a substance; hard to undo ...
Specification
... Symbols, nomenclature, spelling and formatting will follow current IUPAC conventions. These are shown in the reference sheet ‘Quantities, Units, Symbols and Nomenclature used in Chemistry’. This is included with the assessment specifications but will not be provided in the examination. Special notes ...
... Symbols, nomenclature, spelling and formatting will follow current IUPAC conventions. These are shown in the reference sheet ‘Quantities, Units, Symbols and Nomenclature used in Chemistry’. This is included with the assessment specifications but will not be provided in the examination. Special notes ...
Ab Initio Quantum Chemistry: Thermochemistry and Kinetics
... We can use these tools effectively to teach the chemical principles our students will need. • Build on students’ chemistry education and their prior use of properties to solve problems. • Refresh their recognitions of molecule types using ...
... We can use these tools effectively to teach the chemical principles our students will need. • Build on students’ chemistry education and their prior use of properties to solve problems. • Refresh their recognitions of molecule types using ...
Balancing Equations
... stoichiometric coefficients The letters (s), (g), and (l) are the physical states of compounds. ...
... stoichiometric coefficients The letters (s), (g), and (l) are the physical states of compounds. ...
Chemistry Post-Enrolment Worksheet C
... When dealing with compounds we can calculate the percentage by mass of each element that it contains. For example, carbon dioxide contains carbon and oxygen: ...
... When dealing with compounds we can calculate the percentage by mass of each element that it contains. For example, carbon dioxide contains carbon and oxygen: ...
Unit 1. Materials: Formulating Matter A. How do chemists describe
... Classify each statement as a describing either a physical property (P) or a chemical property (C). [Hint: Decide whether the chemical identity of the material does or does not change when the property is observed.] 1. Pure silver has a high luster (is shiny and reflects light). 2. The surfaces of so ...
... Classify each statement as a describing either a physical property (P) or a chemical property (C). [Hint: Decide whether the chemical identity of the material does or does not change when the property is observed.] 1. Pure silver has a high luster (is shiny and reflects light). 2. The surfaces of so ...
Environmental Science
... show how the likelihood of death varies with age, population ecologists use graphs called _______________ ___________. There are 3 types of survivorship curves: I: II: III: ...
... show how the likelihood of death varies with age, population ecologists use graphs called _______________ ___________. There are 3 types of survivorship curves: I: II: III: ...
Herpetological Review Natural History Notes Formatting Guidelines
... in an attempt to standardize key words used for other notes about a similar topic. The section’s intent is to convey information rather than demonstrate prose. A common problem is inclusion of extraneous information. Use of figures is encouraged but they should replace words rather than embellish th ...
... in an attempt to standardize key words used for other notes about a similar topic. The section’s intent is to convey information rather than demonstrate prose. A common problem is inclusion of extraneous information. Use of figures is encouraged but they should replace words rather than embellish th ...
Chapter 3
... 1. Species- group of organisms that can breed & produce fertile offspring 2. Population - groups of organisms of same species living in same area 3. Community - groups of different populations living in same area 4. Ecosystem - all organisms that live in same area along with environment 5. Biome - g ...
... 1. Species- group of organisms that can breed & produce fertile offspring 2. Population - groups of organisms of same species living in same area 3. Community - groups of different populations living in same area 4. Ecosystem - all organisms that live in same area along with environment 5. Biome - g ...
Name - Juan Diego Academy
... Caution: Hot glass looks just like cool glass. Once a test tube has been heated over an open flame, it may take several minutes for it to cool. Be sure that test tubes are cool before handling them. (Step 11.) Caution: Magnesium is extremely flammable. Keep unused strips away from open flames. (Step ...
... Caution: Hot glass looks just like cool glass. Once a test tube has been heated over an open flame, it may take several minutes for it to cool. Be sure that test tubes are cool before handling them. (Step 11.) Caution: Magnesium is extremely flammable. Keep unused strips away from open flames. (Step ...
8th Grade Ch. 7 Chemical Reactions Study guide
... ____ 31. Each substance written to the right of the arrow in a chemical equation is a ____. A. reactant B. product C. precipitate D. catalyst ____ 32. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants? A. There ...
... ____ 31. Each substance written to the right of the arrow in a chemical equation is a ____. A. reactant B. product C. precipitate D. catalyst ____ 32. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants? A. There ...
Dangerous Goods - `OnGuard®` Safety Training
... Because corrosives may react with non-DG goods or corrode them or their packagings, the corrosives storage area should not be used to store any other dangerous goods. DG licensing is required if the total quantity on site exceeds: * 50 kg/L of PG I * 500 kg/L of PG 11 * 1,000 kg/L of PG 111 Storage ...
... Because corrosives may react with non-DG goods or corrode them or their packagings, the corrosives storage area should not be used to store any other dangerous goods. DG licensing is required if the total quantity on site exceeds: * 50 kg/L of PG I * 500 kg/L of PG 11 * 1,000 kg/L of PG 111 Storage ...
Safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is an important component of product stewardship and occupational safety and health. It is intended to provide workers and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information such as physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. SDS formats can vary from source to source within a country depending on national requirements.SDSs are a widely used system for cataloging information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product. These data sheets can be found anywhere where chemicals are being used.There is also a duty to properly label substances on the basis of physico-chemical, health and/or environmental risk. Labels can include hazard symbols such as the European Union standard black diagonal cross on an orange background, used to denote a harmful substance.A SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting.In some jurisdictions, the SDS is required to state the chemical's risks, safety, and effect on the environment.It is important to use an SDS specific to both country and supplier, as the same product (e.g. paints sold under identical brand names by the same company) can have different formulations in different countries. The formulation and hazard of a product using a generic name (e.g. sugar soap) may vary between manufacturers in the same country.