chem10chp7spr08
... - Therefore the total mass cannot change, and the total mass of the reactants will be the same as the total mass of the products ...
... - Therefore the total mass cannot change, and the total mass of the reactants will be the same as the total mass of the products ...
Turn in Homework to the front! 9/7 Warm Up
... 0.489 m. The package states that the ball has a diameter of 0.5 m. Identify the error and percent error of your measurement. ...
... 0.489 m. The package states that the ball has a diameter of 0.5 m. Identify the error and percent error of your measurement. ...
Chapter 13 Notes
... Next count the number of atoms of each element on both sides of the yields arrow. If the number of atoms of each element on the left side of the arrow equals the number on the right, the equation is balanced and no further changes are needed. If they are not the same, then we balance the equation by ...
... Next count the number of atoms of each element on both sides of the yields arrow. If the number of atoms of each element on the left side of the arrow equals the number on the right, the equation is balanced and no further changes are needed. If they are not the same, then we balance the equation by ...
Section 1 The Nature of Chemical Reactions
... compound always contains the same elements in the same proportions, regardless of how the compound is made or how much of the compound is formed. • Because the law of definite proportions holds true for all chemical substances in all reactions, mole ratios can be derived from balanced equations. • M ...
... compound always contains the same elements in the same proportions, regardless of how the compound is made or how much of the compound is formed. • Because the law of definite proportions holds true for all chemical substances in all reactions, mole ratios can be derived from balanced equations. • M ...
Example - cloudfront.net
... • This is what I’ll constantly be telling you to do if you are stuck and you need my help... “Pick an element to balance. How many are on the left side? How many are on the right side? ___________!!! Fe(OH)3 Fe2O3 + H2O Example • Aluminum is a good choice for outdoor furniture because it reacts wi ...
... • This is what I’ll constantly be telling you to do if you are stuck and you need my help... “Pick an element to balance. How many are on the left side? How many are on the right side? ___________!!! Fe(OH)3 Fe2O3 + H2O Example • Aluminum is a good choice for outdoor furniture because it reacts wi ...
Desalination as a Health Hazard
... antibiotic resistant pathogens and DNA coded for antibiotic resistance that have been contributing to the spread and virulence of these "superbugs" to communities. Health care providers are well aware of the increasing incidence of MSRA and other new antibiotic resistant devastating infections. The ...
... antibiotic resistant pathogens and DNA coded for antibiotic resistance that have been contributing to the spread and virulence of these "superbugs" to communities. Health care providers are well aware of the increasing incidence of MSRA and other new antibiotic resistant devastating infections. The ...
___Mg + ___O ___MgO • Mole : Mole ratio
... 1) What is the percentage yield if 5.50 grams of hydrogen gas reacts with nitrogen gas to form 20.4 grams of ammonia (nitrogen trihydride)? 2) What is the percent yield when 2.37 grams of silver nitrate reacts with sodium hydroxide to produce water, sodium nitrate and 1.55 grams of silver oxide? ...
... 1) What is the percentage yield if 5.50 grams of hydrogen gas reacts with nitrogen gas to form 20.4 grams of ammonia (nitrogen trihydride)? 2) What is the percent yield when 2.37 grams of silver nitrate reacts with sodium hydroxide to produce water, sodium nitrate and 1.55 grams of silver oxide? ...
Chemical Synthesis (sat6)
... E = (M gO ∧ H2 → M g ∧ H2 O) ∧ (C ∧ O2 → CO2 )∧ (CO2 ∧ H2 O → H2 CO3 ) ∧ M gO ∧ H2 ∧ O2 ∧ C F = H2 CO3 The complete model code in LPL for this model is as follows (see [1]): Listing 1: The Model ...
... E = (M gO ∧ H2 → M g ∧ H2 O) ∧ (C ∧ O2 → CO2 )∧ (CO2 ∧ H2 O → H2 CO3 ) ∧ M gO ∧ H2 ∧ O2 ∧ C F = H2 CO3 The complete model code in LPL for this model is as follows (see [1]): Listing 1: The Model ...
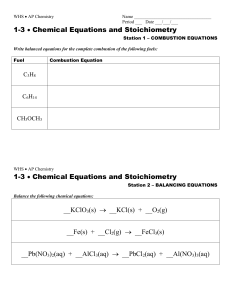
Balancing Chemical Equations
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
UNIT 7 Lecture Notes
... Here are some examples of those equations: • Cu2S + 12 HNO3 Cu(NO3)2 + CuSO4 + 10 NO2 + 6 H2O • 2 K2MnF6 + 4 SbF5 4 KSbF6 + 2 MnF3 + F2 • It’s not one of our objectives that your able to place every single chemical reaction into a specific category, just that you are able to clearly identify the ...
... Here are some examples of those equations: • Cu2S + 12 HNO3 Cu(NO3)2 + CuSO4 + 10 NO2 + 6 H2O • 2 K2MnF6 + 4 SbF5 4 KSbF6 + 2 MnF3 + F2 • It’s not one of our objectives that your able to place every single chemical reaction into a specific category, just that you are able to clearly identify the ...
$doc.title
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
Organismal Biology Study Guide for Test # 4 (4 MAY 2005 – Wed)
... Hominoid = refers to great apes and humans collectively Hominid – more narrow meaning; confined to those twigs of the evolutionary tree that are more colosed related to us than any other species 2 groups of honidis – australopithecines (now extinct) and members of genus Homo Be able to explain misco ...
... Hominoid = refers to great apes and humans collectively Hominid – more narrow meaning; confined to those twigs of the evolutionary tree that are more colosed related to us than any other species 2 groups of honidis – australopithecines (now extinct) and members of genus Homo Be able to explain misco ...
Separation of a Mixture
... A physical change is a change in a materials size, shape, or state of matter, but it is still the same material. It changes its physical appearance but not its composition. A mixture is a combination of different pure substances that still retains its own chemical identity and its own properties ...
... A physical change is a change in a materials size, shape, or state of matter, but it is still the same material. It changes its physical appearance but not its composition. A mixture is a combination of different pure substances that still retains its own chemical identity and its own properties ...
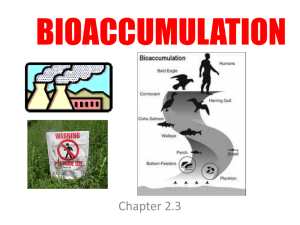
Bioaccumulation
... • Used widely in products like paints, plastics, etc as softening agents from the 1930’s – 1970’s • Banned in North America in 1977 • There are 209 different congeners (different chemical structures) • PCBs interfere with immune function making an organism more susceptible to disease (ex: cancer) ...
... • Used widely in products like paints, plastics, etc as softening agents from the 1930’s – 1970’s • Banned in North America in 1977 • There are 209 different congeners (different chemical structures) • PCBs interfere with immune function making an organism more susceptible to disease (ex: cancer) ...
Physical Property
... point is a physical property (note that the melting point and the freezing point are the same temperature for a given substance). Liquid to Gas Changing from a liquid to a gas is called boiling or vaporization and boiling point is a physical property Gas to Liquid Changing from a gas to a liquid is ...
... point is a physical property (note that the melting point and the freezing point are the same temperature for a given substance). Liquid to Gas Changing from a liquid to a gas is called boiling or vaporization and boiling point is a physical property Gas to Liquid Changing from a gas to a liquid is ...
Student Expectation
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
CHEM 1A General Chemistry I (1)
... Problem solving by application of chemical principles and computation. Preparation of laboratory reports. Reading assigned materials. D. Specific Assignments that Demonstrate Critical Thinking The course emphasizes observation, synthesis of data to arrive at generalizations. Laboratory exercise, rep ...
... Problem solving by application of chemical principles and computation. Preparation of laboratory reports. Reading assigned materials. D. Specific Assignments that Demonstrate Critical Thinking The course emphasizes observation, synthesis of data to arrive at generalizations. Laboratory exercise, rep ...
The Mole Ratio · the ratio between the molar amounts of any two
... · the method of predicting the quantity of a reactant or product in a chemical reaction based on the quantity of another reactant or product in the reaction ...
... · the method of predicting the quantity of a reactant or product in a chemical reaction based on the quantity of another reactant or product in the reaction ...
Reaction Stoichiometry
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
Balancing ANY chemical Equation
... Balanced: EQUAL NUMBERS of atoms for each element involved in the reaction is represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. Law of Conservation of Matter : Matter (atoms) is neither created nor ...
... Balanced: EQUAL NUMBERS of atoms for each element involved in the reaction is represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. Law of Conservation of Matter : Matter (atoms) is neither created nor ...
Chemical Equations and Reaction Types Lab
... equation for a reaction cannot be written unless the substances that are reacting and being formed are both ...
... equation for a reaction cannot be written unless the substances that are reacting and being formed are both ...
I`m providing some explanation of the questions on the exam that
... will be able to purchase the product. ...
... will be able to purchase the product. ...
Chemical reactions
... In some situations, in order not to create confusion, chemical formulas of the reactants and the reaction products are followed by the symbol of the aggregation state written between brackets: ...
... In some situations, in order not to create confusion, chemical formulas of the reactants and the reaction products are followed by the symbol of the aggregation state written between brackets: ...
Ch. 3 9-Station Review
... Solve the following problem: Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
... Solve the following problem: Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
GREEN SEAL CERTIFICATION CHECKLIST
... demonstrate that the product mixture complies. Data from the RTECS and from the HSDB will be accepted, as well as peer-reviewed primary data. For the purposes of estimating the potential toxicity of the chemical mixture, it is assumed that the toxicity of the individual component compounds is additi ...
... demonstrate that the product mixture complies. Data from the RTECS and from the HSDB will be accepted, as well as peer-reviewed primary data. For the purposes of estimating the potential toxicity of the chemical mixture, it is assumed that the toxicity of the individual component compounds is additi ...
Safety data sheet
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is an important component of product stewardship and occupational safety and health. It is intended to provide workers and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information such as physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. SDS formats can vary from source to source within a country depending on national requirements.SDSs are a widely used system for cataloging information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product. These data sheets can be found anywhere where chemicals are being used.There is also a duty to properly label substances on the basis of physico-chemical, health and/or environmental risk. Labels can include hazard symbols such as the European Union standard black diagonal cross on an orange background, used to denote a harmful substance.A SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting.In some jurisdictions, the SDS is required to state the chemical's risks, safety, and effect on the environment.It is important to use an SDS specific to both country and supplier, as the same product (e.g. paints sold under identical brand names by the same company) can have different formulations in different countries. The formulation and hazard of a product using a generic name (e.g. sugar soap) may vary between manufacturers in the same country.